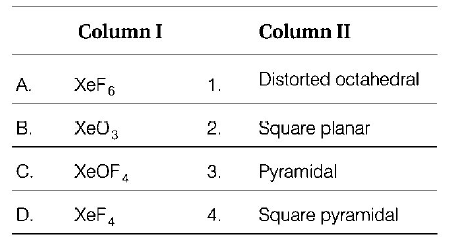
Question
Match the compounds given in column I with the hybridisation and shape given in Column II and mark the correct option.
Match the compounds given in column I with the hybridisation and shape given in Column II and mark the correct option.
Codes
$$\eqalign{
& \,\,\,\,\,\,\,\,\,\,\,{\text{A}}\,\,\,\,\,{\text{B}}\,\,\,\,\,{\text{C}}\,\,\,\,\,{\text{D}} \cr
& \left( {\text{a}} \right)\,\,\,1\,\,\,\,\,\,\,{\text{2}}\,\,\,\,\,\,{\text{4}}\,\,\,\,\,\,{\text{3}} \cr
& \left( {\text{b}} \right)\,\,{\text{4}}\,\,\,\,\,\,\,\,{\text{3}}\,\,\,\,\,\,{\text{1}}\,\,\,\,\,\,{\text{2}} \cr
& \left( {\text{c}} \right)\,\,{\text{4}}\,\,\,\,\,\,\,\,{\text{1}}\,\,\,\,\,\,{\text{2}}\,\,\,\,\,\,{\text{3}} \cr
& \left( {\text{d}} \right)\,\,{\text{1}}\,\,\,\,\,\,\,\,{\text{3}}\,\,\,\,\,\,{\text{4}}\,\,\,\,\,\,{\text{2}} \cr} $$
A.
(a)
B.
(b)
C.
(c)
D.
(d)
Answer :
(d)
Solution :
\[A-1,B-3,C-4,D-2\]
The structure of the xenon compounds are represented below :
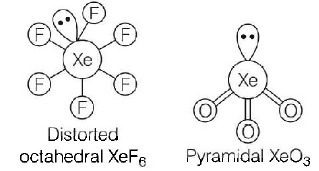
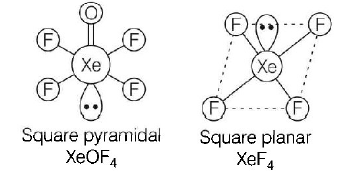
\[A-1,B-3,C-4,D-2\]
The structure of the xenon compounds are represented below :

