Question
Mark the correct order of decreasing acid strength of the following compounds.
Mark the correct order of decreasing acid strength of the following compounds.
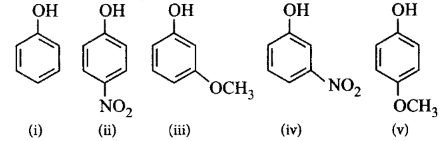
A.
(v) > (iv) > (ii) > (i) > (iii)
B.
(ii) > (iv) > (i) > (iii) > (v)
C.
(iv) > (v) > (iii) > (ii) > (i)
D.
(v) > (iv) > (iii) > (ii) > (i)
Answer :
(ii) > (iv) > (i) > (iii) > (v)
Solution :
The electron withdrawing group $$\left( { - N{O_2}} \right)$$ increases the acidity of phenols and the electron donating group $$\left( { - OC{H_3}} \right)$$ decreases the acidity of phenols. The effect at $$p$$ - position is greater than at $$m$$ - position.
The electron withdrawing group $$\left( { - N{O_2}} \right)$$ increases the acidity of phenols and the electron donating group $$\left( { - OC{H_3}} \right)$$ decreases the acidity of phenols. The effect at $$p$$ - position is greater than at $$m$$ - position.