Question
Mark out the most unlike form of polymerization of \[C{{H}_{2}}=CH-CH=C{{H}_{2}}\]
A.
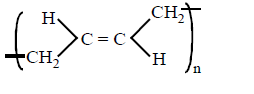

B.
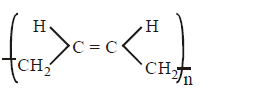
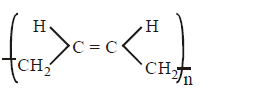
C.
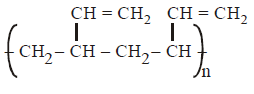
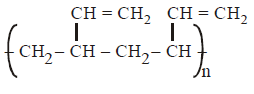
D.
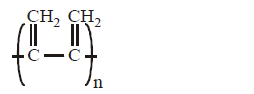
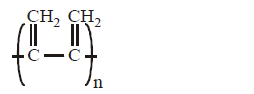
Answer :
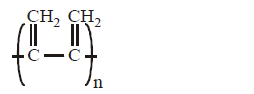
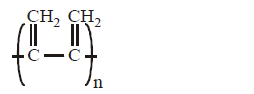
Solution :
In polymerisation of 1, 3 - butadiene either 1, 4 - polymerisation or 1, 2 - polymerisation occurs. In case of 1, 4 - polymerisation, the double bond shifts at \[{{C}_{2}}\] and \[{{C}_{4}}\] carbon, while the chain propagates from \[{{C}_{1}}\] and \[{{C}_{4}}\] end. In this either trans or $$cis$$ polymeric chain is formed.
Option (A) and (B) represent $$'trans'$$ and $$'cis'$$ 1, 4 polymerisation respectively. Option (C) resembles 1, 2 polymerisation, where as option (D) most unlikely to happen.
In polymerisation of 1, 3 - butadiene either 1, 4 - polymerisation or 1, 2 - polymerisation occurs. In case of 1, 4 - polymerisation, the double bond shifts at \[{{C}_{2}}\] and \[{{C}_{4}}\] carbon, while the chain propagates from \[{{C}_{1}}\] and \[{{C}_{4}}\] end. In this either trans or $$cis$$ polymeric chain is formed.
Option (A) and (B) represent $$'trans'$$ and $$'cis'$$ 1, 4 polymerisation respectively. Option (C) resembles 1, 2 polymerisation, where as option (D) most unlikely to happen.