Question
IUPAC name of the compound
IUPAC name of the compound
 is
is
A.
$$trans-3- iodo-4-chloro-3-pentene$$
B.
$$cis-2-chloro-3-iodo-2-pentene$$
C.
$$trans-2-chloro-3-iodo-2-pentene$$
D.
$$cis-3-iodo-4-chloro-3-pentene$$
Answer :
$$trans-2-chloro-3-iodo-2-pentene$$
Solution :
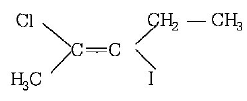
In this compound groups preferential order is $$ - Cl > - C{H_3}\,{\text{and}}\, - I > - C{H_2} - C{H_3}$$
Hence, more preferential order containing groups are attached at opposite sides. So, it is $$E$$ $$(trans)$$ - isomer. Thus, its name is $$trans-2-chloro-3-iodo-2-pentene.$$

In this compound groups preferential order is $$ - Cl > - C{H_3}\,{\text{and}}\, - I > - C{H_2} - C{H_3}$$
Hence, more preferential order containing groups are attached at opposite sides. So, it is $$E$$ $$(trans)$$ - isomer. Thus, its name is $$trans-2-chloro-3-iodo-2-pentene.$$