Question
Isomers of hexane, based on their branching, can be divided
into three distinct classes as shown in the figure.
Isomers of hexane, based on their branching, can be divided
into three distinct classes as shown in the figure.
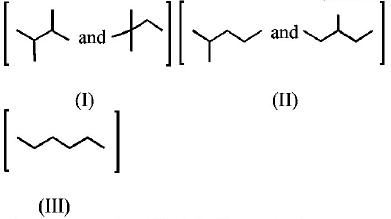
The correct order of their boiling point is
A.
I > II > III
B.
III > II > I
C.
II > III > I
D.
III > I > II
Answer :
III > II > I
Solution :
Greater the extent of branching, lesser is the boiling point of the hydrocarbon, so order of b.p is III > II > I.
Greater the extent of branching, lesser is the boiling point of the hydrocarbon, so order of b.p is III > II > I.