Question
Ionic species are stabilised by the dispersal of charge. Which of the following carboxylate ion is the most stable?
A.
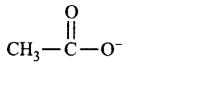
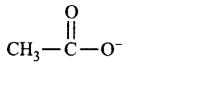
B.
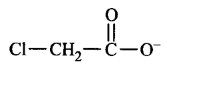
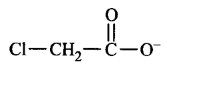
C.


D.


Answer :


Solution :
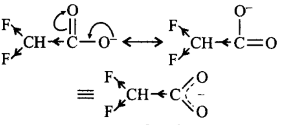
As $$F$$ is most electronegative and in this structure there are two $$F$$ atoms, therefore, dispersal of negative charge is maximum hence it is the most stable.

As $$F$$ is most electronegative and in this structure there are two $$F$$ atoms, therefore, dispersal of negative charge is maximum hence it is the most stable.