Question
In $$Xe{F_2},\,Xe{F_4},Xe{F_6}$$ the number of lone pairs on $$Xe$$ are respectively
A.
$$2,3,1$$
B.
$$1,2,3$$
C.
$$4,1,2$$
D.
$$3,2,1$$
Answer :
$$3,2,1$$
Solution :
In the formation of $$Xe{F_2},s{p^3}d$$ hybridisation occurs which gives the molecule a trigonal bipyramidal structure.
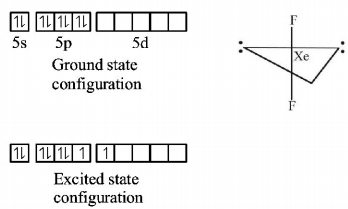
In the formation of $$Xe{F_4},s{p^3}{d^2}$$ hybridization occurs which gives the molecule an octahedral structure.
.PNG)
In the formation of $$Xe{F_6},s{p^3}{d^3}$$ hybridization oocurs which gives the molecule a pentagonal bipyramidal structure.
.PNG)
In the formation of $$Xe{F_2},s{p^3}d$$ hybridisation occurs which gives the molecule a trigonal bipyramidal structure.

In the formation of $$Xe{F_4},s{p^3}{d^2}$$ hybridization occurs which gives the molecule an octahedral structure.
.PNG)
In the formation of $$Xe{F_6},s{p^3}{d^3}$$ hybridization oocurs which gives the molecule a pentagonal bipyramidal structure.
.PNG)