Question
In which of the following molecules/ions $$B{F_3},NO_2^ - ,NH_2^ - \,{\text{and}}\,{H_2}O,$$ the central atom is $$s{p^2}$$ hybridised?
A.
$$NO_2^ - \,{\text{and}}\,NH_2^ - $$
B.
$$NH_2^ - \,{\text{and}}\,{H_2}O$$
C.
$$NO_2^ - \,{\text{and}}\,{H_2}O$$
D.
$$B{F_3}\,{\text{and}}\,NO_2^ - $$
Answer :
$$B{F_3}\,{\text{and}}\,NO_2^ - $$
Solution :
$$B{F_3}$$
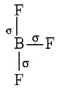 $$ \Rightarrow 3\sigma $$ - bonds, i.e. $$s{p^2}$$ hybridisation
$$ \Rightarrow 3\sigma $$ - bonds, i.e. $$s{p^2}$$ hybridisation
Planar structure
$$NO_2^ - $$
 $$ \Rightarrow 2\sigma $$ - bonds $$+1$$ lone pair of
electrons, i.e. $$s{p^2}$$ hybridisation
$$ \Rightarrow 2\sigma $$ - bonds $$+1$$ lone pair of
electrons, i.e. $$s{p^2}$$ hybridisation
$$NH_2^ - $$
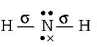 $$ \Rightarrow 2\sigma $$ - bonds $$+2$$ lone pairs, i.e. $$s{p^3}$$ hybridisation
$$ \Rightarrow 2\sigma $$ - bonds $$+2$$ lone pairs, i.e. $$s{p^3}$$ hybridisation

$$ \Rightarrow 2\sigma $$ - bonds $$+2$$ lone pairs, i.e. $$s{p^3}$$ hybridisation, Thus, in $$B{F_3}$$ and $$NO_2^ - ,$$ central atom is $$s{p^2}$$ hybridised, while $$N{H_2},N{H_3}$$ and $${H_2}O$$ are $$s{p^3}$$ hybridised.
$$B{F_3}$$
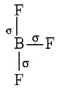 $$ \Rightarrow 3\sigma $$ - bonds, i.e. $$s{p^2}$$ hybridisation
$$ \Rightarrow 3\sigma $$ - bonds, i.e. $$s{p^2}$$ hybridisation Planar structure
$$NO_2^ - $$
 $$ \Rightarrow 2\sigma $$ - bonds $$+1$$ lone pair of
electrons, i.e. $$s{p^2}$$ hybridisation
$$ \Rightarrow 2\sigma $$ - bonds $$+1$$ lone pair of
electrons, i.e. $$s{p^2}$$ hybridisation $$NH_2^ - $$
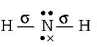 $$ \Rightarrow 2\sigma $$ - bonds $$+2$$ lone pairs, i.e. $$s{p^3}$$ hybridisation
$$ \Rightarrow 2\sigma $$ - bonds $$+2$$ lone pairs, i.e. $$s{p^3}$$ hybridisation 
$$ \Rightarrow 2\sigma $$ - bonds $$+2$$ lone pairs, i.e. $$s{p^3}$$ hybridisation, Thus, in $$B{F_3}$$ and $$NO_2^ - ,$$ central atom is $$s{p^2}$$ hybridised, while $$N{H_2},N{H_3}$$ and $${H_2}O$$ are $$s{p^3}$$ hybridised.