Question
In which of the following molecules are all the bonds not equal?
A.
$$Cl{F_3}$$
B.
$$B{F_3}$$
C.
$$Al{F_3}$$
D.
$$N{F_3}$$
Answer :
$$Cl{F_3}$$
Solution :
In $$Cl{F_3}$$ all bonds are not equal due to its trigonal-bipyramidal ($$s{p^3}d$$ hybridisation) geometry

$$B{F_3}$$ and $$Al{F_3}$$ show trigonal symmetric structure due to $$s{p^2}$$ hybridisation.
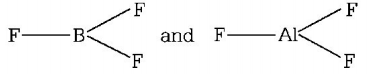
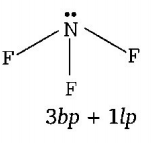
$$N{F_3}$$ shows pyramidal geometry due to $$s{p^3}$$ hybridisation.
In $$Cl{F_3}$$ all bonds are not equal due to its trigonal-bipyramidal ($$s{p^3}d$$ hybridisation) geometry

$$B{F_3}$$ and $$Al{F_3}$$ show trigonal symmetric structure due to $$s{p^2}$$ hybridisation.
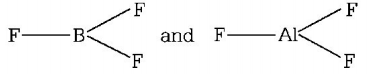
$$N{F_3}$$ shows pyramidal geometry due to $$s{p^3}$$ hybridisation.
