Question
In the presence of peroxide, hydrogen chloride and hydrogen iodide do not give anti-Markovnikov addition to alkenes because
A.
both are highly ionic
B.
one is oxidizing and the other is reducing
C.
one of the steps is endothermic in both the cases
D.
all the steps are exothermic in both the cases
Answer :
one of the steps is endothermic in both the cases
Solution :
TIPS/Formulae :
Peroxide effect is effective only in case of $$HBr$$ and not in case of $$HCl$$ and $$HI.$$
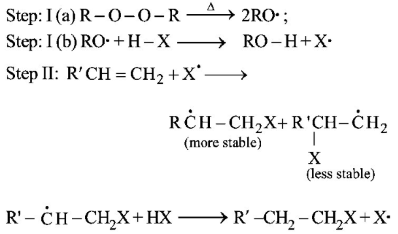
For $$HCl,$$ Step-I (b) is endothermic while step-II is exothermic but for $$HI,$$ Step-I (b) is exothermic while Step-II is endothermic.
TIPS/Formulae :
Peroxide effect is effective only in case of $$HBr$$ and not in case of $$HCl$$ and $$HI.$$

For $$HCl,$$ Step-I (b) is endothermic while step-II is exothermic but for $$HI,$$ Step-I (b) is exothermic while Step-II is endothermic.