Question
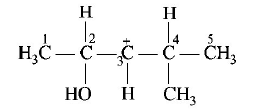
In the following carbocation, $$H/C{H_3}$$ that is most likely to migrate to the positively charged carbon is
In the following carbocation, $$H/C{H_3}$$ that is most likely to migrate to the positively charged carbon is
A.
$$C{H_3}\,{\text{at}}\,C - 4$$
B.
$$H\,{\text{at}}\,C - 4$$
C.
$$C{H_3}\,{\text{at}}\,C - 2$$
D.
$$H\,{\text{at}}\,C - 2$$
Answer :
$$H\,{\text{at}}\,C - 2$$
Solution :
NOTE: Migrating tendency of hydride is greater than that of alkyl group. Further migration of hydride from $$C - 2$$ gives more stable carbocation ( stabilized by $$ + R$$ effect of $$OH$$ group and $$ + I$$ and hyper conjugative effects of methyl group ).

NOTE: Migrating tendency of hydride is greater than that of alkyl group. Further migration of hydride from $$C - 2$$ gives more stable carbocation ( stabilized by $$ + R$$ effect of $$OH$$ group and $$ + I$$ and hyper conjugative effects of methyl group ).
