Question
In $$Br{F_3}$$ molecule, the lone pairs occupy equatorial positions to minimize
A.
lone pair - bond pair repulsion only
B.
bond pair - bond pair repulsion only
C.
lone pair - lone pair repulsion and lone pair - bond pair repulsion
D.
lone pair - lone pair repulsion only
Answer :
lone pair - lone pair repulsion and lone pair - bond pair repulsion
Solution :
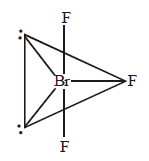
In $$Br{F_3},$$ both bond pairs as well as lone pairs of electrons are present. Due to the presence of lone pairs of electrons $$(lp)$$ in the valence shell, the bond angle is contracted and the molecule takes the $$T$$ - shape. This is due to greater repulsion between two lone pairs or between a lone pair and a bond pair than between the two bond pairs.

In $$Br{F_3},$$ both bond pairs as well as lone pairs of electrons are present. Due to the presence of lone pairs of electrons $$(lp)$$ in the valence shell, the bond angle is contracted and the molecule takes the $$T$$ - shape. This is due to greater repulsion between two lone pairs or between a lone pair and a bond pair than between the two bond pairs.