Question
In $$Br{F_3}$$ molecule, the lone pairs occupy equatorial positions to minimise
A.
lone pair-bond pair repulsion
B.
bond pair-bond pair repulsion
C.
lone pair-lone pair repulsion and lone pair-bond pair repulsion
D.
lone pair-lone pair repulsion
Answer :
lone pair-lone pair repulsion
Solution :
In $$Br{F_3}$$ molecule, $$Br$$ is $$s{p^3}d$$ hybridised, but its geometry is $$T$$-shaped due to distortion of geometry from trigonal bipyramidal to $$T$$-shaped by the involvement of lone pair-lone pair repulsion.
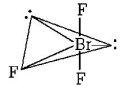
Here, $$lp - lp\,\,{\text{repulsion}} = 0$$
$$lp - bp\,\,{\text{repulsion}} = 4$$
$$bp - bp\,\,{\text{repulsion}} = 2$$
In $$Br{F_3}$$ molecule, $$Br$$ is $$s{p^3}d$$ hybridised, but its geometry is $$T$$-shaped due to distortion of geometry from trigonal bipyramidal to $$T$$-shaped by the involvement of lone pair-lone pair repulsion.

Here, $$lp - lp\,\,{\text{repulsion}} = 0$$
$$lp - bp\,\,{\text{repulsion}} = 4$$
$$bp - bp\,\,{\text{repulsion}} = 2$$