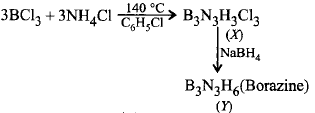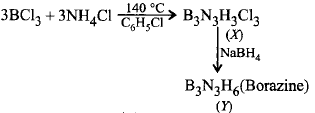Question
Identify $$X$$ and $$Y$$ in the following reaction : \[BC{{l}_{3}}+N{{H}_{4}}Cl\xrightarrow[{{C}_{6}}{{H}_{5}}Cl]{{{140}^{\circ }}C}X\] \[\xrightarrow{NaB{{H}_{4}}}Y\]
A.
$$X = NaB{O_2},Y = {B_2}{O_3}$$
B.
$$X = N{a_2}{B_4}{O_7},Y = {H_3}B{O_3}$$
C.
$$X = BN,Y = {\left[ {N{H_4}} \right]^ + }{\left[ {BC{l_4}} \right]^ - }$$
D.
$$X = {B_3}{N_3}{H_3}C{l_3},Y = {B_3}{N_3}{H_6}$$
Answer :
$$X = {B_3}{N_3}{H_3}C{l_3},Y = {B_3}{N_3}{H_6}$$
Solution :