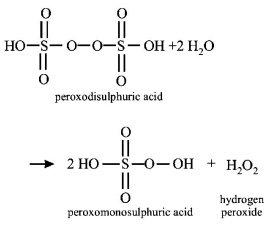
Question
Hydrolysis of one mole of peroxodisulphuric acid produces
A.
two moles of sulphuric acid
B.
two moles of peroxomonosulphuric acid
C.
one mole of sulphuric acid and one mole of peroxomonosulphuric acid
D.
one mole of sulphuric acid, one mole of
peroxomonosulphuric acid and one mole of hydrogen peroxide.
Answer :
one mole of sulphuric acid, one mole of
peroxomonosulphuric acid and one mole of hydrogen peroxide.
Solution :