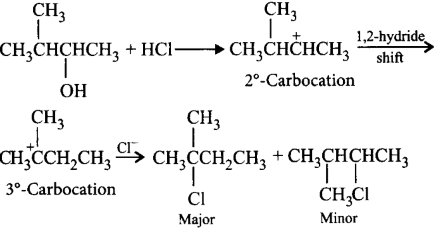
Question
Halogen acids react with alcohols to form alkyl halides. The reaction follows a nucleophilic substitution mechanism. What will be the major product of the following reaction?
Halogen acids react with alcohols to form alkyl halides. The reaction follows a nucleophilic substitution mechanism. What will be the major product of the following reaction?
\[C{{H}_{3}}\overset{\begin{smallmatrix}
\,\,C{{H}_{3}} \\
|\,\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\,\,\, \\
OH\,\,\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{CH-}}\,C{{H}_{3}}+HCl\to \]
A.
\[\underset{\begin{smallmatrix}
| \\
\,\,\,C{{H}_{3}}
\end{smallmatrix}}{\mathop{C{{H}_{3}}CH-}}\,\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\,\,\,\, \\
Cl\,\,\,\,\,\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{CH-}}\,C{{H}_{3}}\]
B.
\[\underset{\begin{smallmatrix}
| \\
Cl
\end{smallmatrix}}{\mathop{C{{H}_{3}}CH-}}\,\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\,\,\, \\
C{{H}_{3}}\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{CH-}}\,C{{H}_{3}}\]
C.
\[C{{H}_{3}}\underset{\begin{smallmatrix}
| \\
Cl
\end{smallmatrix}}{\overset{\begin{smallmatrix}
\,\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{-C-}}}\,C{{H}_{2}}C{{H}_{3}}\]
D.
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}Cl\]
Answer :
\[C{{H}_{3}}\underset{\begin{smallmatrix}
| \\
Cl
\end{smallmatrix}}{\overset{\begin{smallmatrix}
\,\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{-C-}}}\,C{{H}_{2}}C{{H}_{3}}\]
Solution :