Question
$${H_3}B{O_3}$$ is:
A.
Monobasic and weak Lewis acid
B.
Monobasic and weak Bronsted acid
C.
Monobasic and strong Lewis acid
D.
Tribasic and weak Bronsted acid
Answer :
Monobasic and weak Lewis acid
Solution :
The central boron atom in boric acid, $${H_3}B{O_3}$$ is electrondeficient.
NOTE : Boric acid is a Lewis acid with one $$p$$ - orbital vacant. There is no $$d$$ - orbital of suitable energy in boron atom. So, it can accommodate only one additional electron pair in its outermost shell.
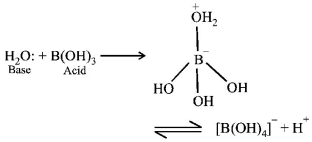
The central boron atom in boric acid, $${H_3}B{O_3}$$ is electrondeficient.
NOTE : Boric acid is a Lewis acid with one $$p$$ - orbital vacant. There is no $$d$$ - orbital of suitable energy in boron atom. So, it can accommodate only one additional electron pair in its outermost shell.
