Question
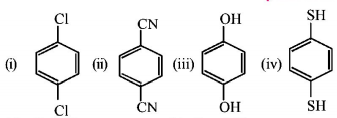
For which of the following molecule significant $$\mu \ne 0?$$
For which of the following molecule significant $$\mu \ne 0?$$
A.
Only (i)
B.
(i) and (ii)
C.
Only (iii)
D.
(iii) and (iv)
Answer :
(iii) and (iv)
Solution :
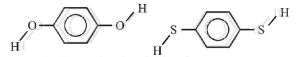
In both the molecules the bond moments are not canceling with each other and hence the molecules has aresultant dipole and hence the molecule is polar.

In both the molecules the bond moments are not canceling with each other and hence the molecules has aresultant dipole and hence the molecule is polar.