Question
For $${H_3}P{O_3}$$ and $${H_3}P{O_4},$$ the correct choice is
A.
$${H_3}P{O_3}$$ is dibasic and reducing
B.
$${H_3}P{O_4}$$ is diabasic and non-reducing
C.
$${H_3}P{O_4}$$ is tribasic and reducing
D.
$${H_3}P{O_4}$$ is tribasic and non reducing
Answer :
$${H_3}P{O_3}$$ is dibasic and reducing
Solution :
The structure of $${H_3}P{O_3}$$ is as given below.
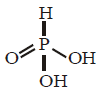
There are only two $$ - OH$$ groups and hence it is dibasic. The oxidation number of $$P$$ in this acid is +3, whereas $$P$$ may have +5 oxidation state also. Therefore, $${H_3}P{O_3}$$ can be oxidised which means $${H_3}P{O_3}$$ is a reducing agent.
The structure of $${H_3}P{O_3}$$ is as given below.

There are only two $$ - OH$$ groups and hence it is dibasic. The oxidation number of $$P$$ in this acid is +3, whereas $$P$$ may have +5 oxidation state also. Therefore, $${H_3}P{O_3}$$ can be oxidised which means $${H_3}P{O_3}$$ is a reducing agent.