Question
For an endothermic reaction, energy of activation is $${E_a}$$ and enthalpy of reaction is $$\Delta H$$ ( both of these in $$kJ/mol$$ ). Minimum value of $${E_a}$$ will be
A.
less than $$\Delta H$$
B.
equal to $$\Delta H$$
C.
more than $$\Delta H$$
D.
equal to zero
Answer :
more than $$\Delta H$$
Solution :
Key Idea In endothermic reactions, energy of reactants is less than that of the products.
Potential energy diagram for endothermic reactions is
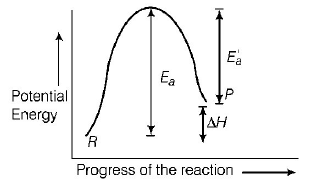
where,
$${E_a} = $$ activation energy of forward reaction
$${{E'}_a} = $$ activation energy of backward reaction
$$\Delta H = $$ enthalpy of the reaction.
From the above diagram,
$$\eqalign{ & {E_a} = {{E'}_a} + \Delta H \cr & {\text{Thus,}}\,\,{E_a} > \Delta H \cr} $$
Key Idea In endothermic reactions, energy of reactants is less than that of the products.
Potential energy diagram for endothermic reactions is

where,
$${E_a} = $$ activation energy of forward reaction
$${{E'}_a} = $$ activation energy of backward reaction
$$\Delta H = $$ enthalpy of the reaction.
From the above diagram,
$$\eqalign{ & {E_a} = {{E'}_a} + \Delta H \cr & {\text{Thus,}}\,\,{E_a} > \Delta H \cr} $$