Question
For $$1$$ $$mol$$ of an ideal gas at a constant temperature $$T,$$ the plot of $$log\,P$$ against $$log\,V$$ is a ( $$P$$ : Pressure, $$V$$ : Volume )
A.
Straight line parallel to $$x$$ - axis.
B.
Straight line with a negative slope.
C.
Curve starting at origin.
D.
Straight line passing through origin.
Answer :
Straight line with a negative slope.
Solution :
According to Boyle's law, $$PV=$$ constant
$$\eqalign{ & \therefore \,\,{\text{log}}\,P + {\text{log}}\,V = {\text{constant}} \cr & \,{\text{log}}\,P = - {\text{log}}\,V + {\text{constant}} \cr} $$
Hence, the plot of $${\text{log}}\,P\,\,{\text{vs}}\,\,{\text{log}}\,V$$ is straight line with negative slope.
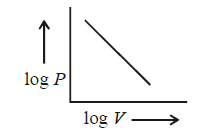
According to Boyle's law, $$PV=$$ constant
$$\eqalign{ & \therefore \,\,{\text{log}}\,P + {\text{log}}\,V = {\text{constant}} \cr & \,{\text{log}}\,P = - {\text{log}}\,V + {\text{constant}} \cr} $$
Hence, the plot of $${\text{log}}\,P\,\,{\text{vs}}\,\,{\text{log}}\,V$$ is straight line with negative slope.
