Question
Ethanol and dimethyl ether form a pair of functional isomers. The boiling point of ethanol is higher than that of dimethyl ether due to the presence of
A.
$$H$$ - bonding in ethanol
B.
$$H$$ - bonding in dimethyl ether
C.
$$ - C{H_3}$$ group in ethanol
D.
$$ - C{H_3}$$ group in dimethyl ether
Answer :
$$H$$ - bonding in ethanol
Solution :
Alcohols have higher boiling points as compared to other organic compounds of similar molecular masses such as ethers. This is due to the presence of intermolecular hydrogen bonding in alcohols which is absent in ethers. Because of hydrogen bonding in alcohols, these exist as associated molecules rather than discrete molecules. Consequently, a large amount of energy is required to break these bonds and therefore, their boiling points are high.
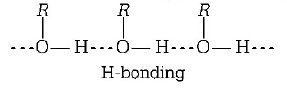
Alcohols have higher boiling points as compared to other organic compounds of similar molecular masses such as ethers. This is due to the presence of intermolecular hydrogen bonding in alcohols which is absent in ethers. Because of hydrogen bonding in alcohols, these exist as associated molecules rather than discrete molecules. Consequently, a large amount of energy is required to break these bonds and therefore, their boiling points are high.
