Question
Dipole moment of $${H_2}O$$ is $$1.85\,D.$$ If the bond angle is $${105^ \circ }$$ and $$O - H$$ bond length is $$0.94\mathop {\text{A}}\limits^{\text{o}} ,$$ what is the magnitude of charge on the oxygen atom in water molecule?
A.
$$2 \times {10^{ - 10}}esu$$
B.
$$4.28 \times {10^{ - 10}}esu$$
C.
$$3.22 \times {10^{ - 10}}esu$$
D.
$$1.602 \times {10^{ - 19}}C$$
Answer :
$$3.22 \times {10^{ - 10}}esu$$
Solution :
$$\mu = 1.85\,D = 1.85 \times {10^{ - 18}}esu\,cm = q \times d$$
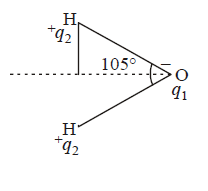
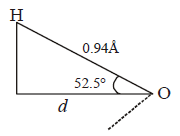
$$\eqalign{ & \cos \,\,{52.5^ \circ } = \frac{d}{{0.94\mathop {\text{A}}\limits^{\text{o}} }} \Rightarrow d = 0.609 \times 0.94\mathop {\text{A}}\limits^{\text{o}} \cr & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\, = 0.572\mathop {\text{A}}\limits^{\text{o}} \cr & \therefore \mu = q \times d \cr & {q_1} = \frac{\mu }{d} = \frac{{1.85D}}{{0.572\mathop {\text{A}}\limits^{\text{o}} }} \cr & = \frac{{1.85 \times {{10}^{ - 18}}esu\,cm}}{{0.572 \times {{10}^{ - 8}}cm}} \cr & = 3.2 \times {10^{ - 10}}esu \cr} $$
$$\mu = 1.85\,D = 1.85 \times {10^{ - 18}}esu\,cm = q \times d$$


$$\eqalign{ & \cos \,\,{52.5^ \circ } = \frac{d}{{0.94\mathop {\text{A}}\limits^{\text{o}} }} \Rightarrow d = 0.609 \times 0.94\mathop {\text{A}}\limits^{\text{o}} \cr & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\, = 0.572\mathop {\text{A}}\limits^{\text{o}} \cr & \therefore \mu = q \times d \cr & {q_1} = \frac{\mu }{d} = \frac{{1.85D}}{{0.572\mathop {\text{A}}\limits^{\text{o}} }} \cr & = \frac{{1.85 \times {{10}^{ - 18}}esu\,cm}}{{0.572 \times {{10}^{ - 8}}cm}} \cr & = 3.2 \times {10^{ - 10}}esu \cr} $$