Question
Dehydration of alcohols by \[conc.\,{{H}_{2}}S{{O}_{4}}\] takes place according to following steps :
Dehydration of alcohols by \[conc.\,{{H}_{2}}S{{O}_{4}}\] takes place according to following steps :
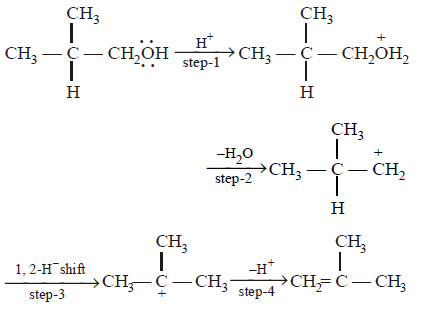
The slowest and fastest steps in the above reaction are
A.
step 1 is slowest, while 3 is fastest.
B.
step 2 is slowest, while 3 is fastest.
C.
step 2 is slowest, while 4 is fastest.
D.
all steps proceed at equal rate.
Answer :
step 2 is slowest, while 4 is fastest.
Solution :
Step 2 involves the formation of carbonium ion by the loss of weakly basic \[{{H}_{2}}O\] molecule. It is slowest step. Step 4 involves the conversion of an unstable ( or intermediate ) into a quite stable product, hence it is fastest step.
Step 2 involves the formation of carbonium ion by the loss of weakly basic \[{{H}_{2}}O\] molecule. It is slowest step. Step 4 involves the conversion of an unstable ( or intermediate ) into a quite stable product, hence it is fastest step.