Question
Consider the two figures given below.
Consider the two figures given below.
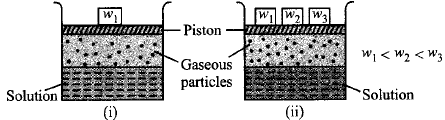
Which of the following statements regarding the experiment is true ?
A.
The solubility of a gas in liquid in beaker (i) is greater than that in beaker (ii),
B.
The solubility of a gas in beaker (i) is less than that in beaker (ii).
C.
The solubility of a gas is equal in both beakers.
D.
The solubility of a gas remains unaffected by change in weights.
Answer :
The solubility of a gas in beaker (i) is less than that in beaker (ii).
Solution :
The solubility of gas in a liquid increases with increase in pressure and is directly proportional to the pressure of the gas.
The solubility of gas in a liquid increases with increase in pressure and is directly proportional to the pressure of the gas.