Question
Consider the reactions,
Consider the reactions,

Identify $$A, X,Y$$ and $$Z$$
A.
$$A$$ - methoxymethane, $$X$$ - ethanoic acid, $$Y$$ - acetate ion, $$Z$$ - hydrazine
B.
$$A$$ - methoxymethane, $$X$$ - ethanol, $$Y$$ - ethanoic acid, $$Z$$ - semicarbazide
C.
$$A$$ - ethanal, $$X$$ - Acetaldelyde, $$Y$$ - but - 2 - enal, $$Z$$ - semicarbazone
D.
$$A$$ - ethanol, $$X$$ - acetaldehyde, $$Y$$ - butanone, $$Z$$ - hydrazone
Answer :
$$A$$ - ethanal, $$X$$ - Acetaldelyde, $$Y$$ - but - 2 - enal, $$Z$$ - semicarbazone
Solution :
Aldehydes gives silver minor test so, $$'X’$$ may be alcohol which is oxidised by $$Cu$$ gives aldehydes.
Therefore,
A is acetaldehyde $$\left( {C{H_3}CHO} \right)$$
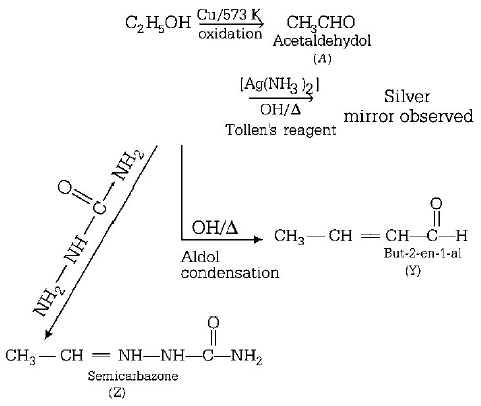
Aldehydes gives silver minor test so, $$'X’$$ may be alcohol which is oxidised by $$Cu$$ gives aldehydes.
Therefore,
A is acetaldehyde $$\left( {C{H_3}CHO} \right)$$
