Question
Consider the reaction, $$A \to B.$$ The concentration of both the reactants and the products varies exponentially with time. Which of the following figures correctly describes the change in concentration of reactants and products with time?
A.
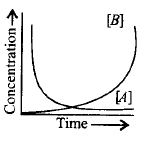

B.


C.
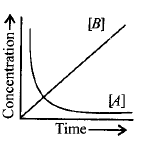

D.


Answer :


Solution :
In a reaction $$A \to B,$$ concentration of reactant decreases as concentration of product increases during the course of a reaction.
In a reaction $$A \to B,$$ concentration of reactant decreases as concentration of product increases during the course of a reaction.