Question
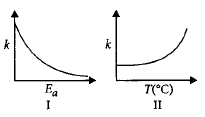
Consider the given plots for a reaction obeying Arrhenius equation $$\left( {{0^ \circ }C < T < 300{\,^ \circ }C} \right):$$ ( $$k$$ and $${E_a}$$ are rate constant and activation energy, respectively )
Choose the correct option.
A.
I is right but II is wrong.
B.
Both I and II are wrong.
C.
I is wrong but II is right.
D.
Both I and II are correct.
Answer :
Both I and II are correct.
Solution :
According to Arrhenius equation, $$k = A{e^{ - \frac{{Ea}}{{RT}}}}$$
On increasing the value of $${E_a},k$$ is decreasing. So, curve I is correct.
On increasing temperature $$\left( T \right),k$$ is increasing. So, curve II is also correct.
According to Arrhenius equation, $$k = A{e^{ - \frac{{Ea}}{{RT}}}}$$
On increasing the value of $${E_a},k$$ is decreasing. So, curve I is correct.
On increasing temperature $$\left( T \right),k$$ is increasing. So, curve II is also correct.