Question
Consider the following reactions :
Consider the following reactions :
Reaction I :
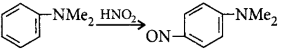
Reaction II :
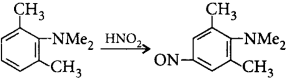
Which of the following is a correct comparison of rate of reaction?
A.
$${r_I} > {r_{II}}$$
B.
$${r_I} < {r_{II}}$$
C.
$${r_I} = {r_{II}}$$
D.
$${\text{Reactions are not possible}}$$
Answer :
$${r_I} > {r_{II}}$$
Solution :
The reactive species is the nitrosonium ion (electrophile) and the most likely site of attack will be that with the highest electron density.
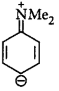
Such kind of delocalisation is not found in 2, 6-dimethyl derivative due to steric inhibition of resonance.
The reactive species is the nitrosonium ion (electrophile) and the most likely site of attack will be that with the highest electron density.

Such kind of delocalisation is not found in 2, 6-dimethyl derivative due to steric inhibition of resonance.