Question
Consider figure and mark the correct option.
Consider figure and mark the correct option.
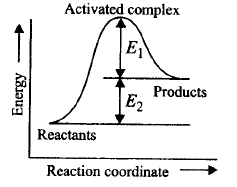
A.
Activation energy of forward reaction is $${E_1} + {E_2}$$ and product is less stable than reactant.
B.
Activation energy of forward reaction is $${E_1} + {E_2}$$ and product is more stable than reactant.
C.
Activation energy of both forward and backward reaction is $${E_1} + {E_2}$$ and reactant is more stable than product.
D.
Activation energy of backward reaction is $${E_1}$$ and product is more stable than reactant.
Answer :
Activation energy of forward reaction is $${E_1} + {E_2}$$ and product is less stable than reactant.
Solution :
$${E_{a\left( {{\text{forward}}\,\,{\text{reaction}}} \right)}} = {E_1} + {E_2}$$ and product is less stable as it has higher energy.
$${E_{a\left( {{\text{forward}}\,\,{\text{reaction}}} \right)}} = {E_1} + {E_2}$$ and product is less stable as it has higher energy.