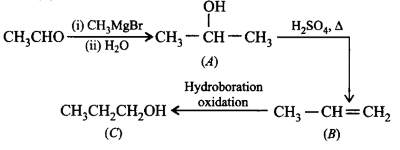
Question
Compounds $$(A)$$ and $$(C)$$ in the following reactions are
Compounds $$(A)$$ and $$(C)$$ in the following reactions are
\[C{H_3}CHO\xrightarrow[{\left( {{\text{ii}}} \right)\,{H_2}O}]{{\left( {\text{i}} \right)\,C{H_3}MgBr}}\left( A \right)\] \[\xrightarrow{{{H_2}S{O_4},\,\Delta }}\left( B \right)\] \[\xrightarrow{{{\text{Hydroboration oxidation}}}}\left( C \right)\]
A.
identical
B.
positional isomers
C.
functional isomers
D.
optical isomers
Answer :
positional isomers
Solution :