Question
Compound $$'A'$$ ( molecular formula $${C_3}{H_8}O$$ ) is treated with acidified potassium dichromate to form a product $$'B'$$ ( molecular formula $${C_3}{H_6}O$$ ). $$'B'$$ forms a shining silver mirror on warming with ammonical silver nitrate. $$'B'$$ when treated with an aqueous solution of $${H_2}NCONHN{H_2}.HCl$$ and sodium acetate gives a product $$'C'.$$ Identify the structure of $$'C'$$
A.
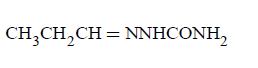
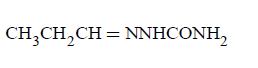
B.


C.
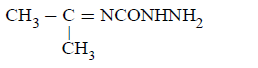
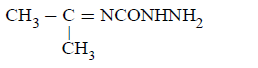
D.
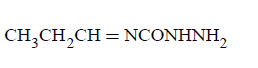
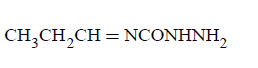
Answer :
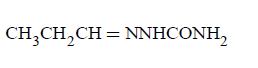
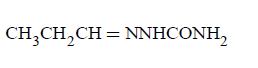
Solution :
\[\underset{A}{\mathop{{{C}_{3}}{{H}_{8}}O\xrightarrow{{{K}_{2}}C{{r}_{2}}{{O}_{7}}/{{H}^{+}}}}}\,\underset{B\left( -CHO \right)}{\mathop{{{C}_{3}}{{H}_{6}}O\xrightarrow{{{H}_{2}}NCONHN{{H}_{2}}}C}}\,\]
Since $$B$$ reduces Tollen’s reagent, it indicates that it has an $$-CHO$$ group, so it must be \[C{{H}_{3}}C{{H}_{2}}CHO.\]
Hence
\[\underset{\left[ A \right]}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH}}\,\to \underset{\left[ B \right]}{\mathop{C{{H}_{3}}C{{H}_{2}}CHO}}\,\] \[\xrightarrow{{{H}_{2}}NNHCON{{H}_{2}}}\underset{\left[ C \right]}{\mathop{C{{H}_{3}}C{{H}_{2}}CH}}\,=\] \[NNHCON{{H}_{2}}\]
\[\underset{A}{\mathop{{{C}_{3}}{{H}_{8}}O\xrightarrow{{{K}_{2}}C{{r}_{2}}{{O}_{7}}/{{H}^{+}}}}}\,\underset{B\left( -CHO \right)}{\mathop{{{C}_{3}}{{H}_{6}}O\xrightarrow{{{H}_{2}}NCONHN{{H}_{2}}}C}}\,\]
Since $$B$$ reduces Tollen’s reagent, it indicates that it has an $$-CHO$$ group, so it must be \[C{{H}_{3}}C{{H}_{2}}CHO.\]
Hence
\[\underset{\left[ A \right]}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH}}\,\to \underset{\left[ B \right]}{\mathop{C{{H}_{3}}C{{H}_{2}}CHO}}\,\] \[\xrightarrow{{{H}_{2}}NNHCON{{H}_{2}}}\underset{\left[ C \right]}{\mathop{C{{H}_{3}}C{{H}_{2}}CH}}\,=\] \[NNHCON{{H}_{2}}\]