151.
van der Waal’s equation for a gas is stated as, $$P = \frac{{nRT}}{{V - nb}} - a{\left( {\frac{n}{V}} \right)^2}.$$
This equation reduces to the perfect gas equation, $$P = \frac{{nRT}}{V}$$ when,
A
temperature is sufficient high and pressure is low.
B
temperature is sufficient low and pressure is high.
C
both temperature and pressure are very high.
D
both temperature and pressure are very low.
Answer :
temperature is sufficient high and pressure is low.
152. The relations between various variables of gaseous substances are given along with their formulae. Mark the incorrect relationship.
A
Density and molar mass $$:M = \frac{{dRT}}{P}$$
B
Universal gas constant, $$P,V,T:R = \frac{{PV}}{{nT}}$$
C
Volume and pressure $$:{V_2} = \frac{{{P_2}{V_1}}}{{{P_1}}}$$
D
Volume and temperature $$:{V_2} = \frac{{{V_1}{T_2}}}{{{T_1}}}$$
Answer :
Volume and pressure $$:{V_2} = \frac{{{P_2}{V_1}}}{{{P_1}}}$$
153. At $${25^ \circ }C$$ and $$730\,mm$$ pressure, $$380\,mL$$ of dry oxygen was collected. If the temperature is constant, what volume will the oxygen occupy at $$760\,mm$$ pressure?
A
365$$\,mL$$
B
2$$\,mL$$
C
10$$\,mL$$
D
20$$\,mL$$
Answer :
365$$\,mL$$
154.
$$X$$ and $$Y$$ are two volatile liquids with molar weights of $$10\,g\,mo{l^{ - 1}}$$ and $$40\,g\,mo{l^{ - 1}}$$ respectively. Two cotton plugs, one soaked in $$X$$ and the other soaked in $$Y,$$ are Simultaneously placed at the ends of a tube of length $$L = 24\,cm,$$ as shown in the figure. The tube is filled with an inert gas at 1atmosphere pressure and a temperature of $$300\,K.$$ Vapours of $$X$$ and $$Y$$ react to form a product which is first observed at a distance $$d\,cm$$ from the plug soaked in $$X.$$ Take $$X$$ and $$Y$$ to have equal molecular diameters and assume ideal behaviour for the inert gas and the vapours.
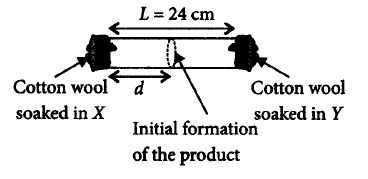
The value of $$d$$ in $$cm$$ ( shown in the figure ), as estimated from Graham's law, is
A
8
B
12
C
16
D
20
Answer :
16
155. A manifestation of surface tension is :
A
rise of liquid in a capillary tube
B
spherical shape of liquid drops
C
upward movement of water in soils
D
All the above
Answer :
All the above
156.
There are three closed containers in which equal amount of the gas are filled.
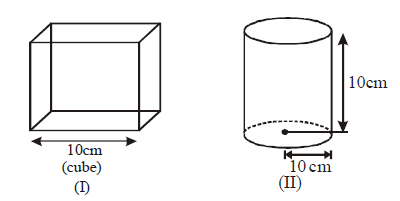
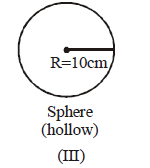
If all the containers are placed at the same temperatures, then find the incorrect options –
A
Pressure of the gas is minimum in (III) container
B
Pressure of the gas is equal in (I) and (II) container
C
Pressure of the gas is maximum in (I)
D
The ratio of pressure in (II) and (III) container is 4 : 3
Answer :
Pressure of the gas is equal in (I) and (II) container
157. A given metal crystallises out with a cubic structure having edge length of $$361\,pm.$$ If there are four metal atoms in one unit cell, what is the radius of one atom?
A
40$$\,pm$$
B
127$$\,pm$$
C
80$$\,pm$$
D
108$$\,pm$$
Answer :
127$$\,pm$$
158. The ionic radii of $${A^ + }$$ and $${B^ - }\,ions$$ are $$0.98 \times {10^{ - 10}}m$$ and $$1.81 \times {10^{ - 10}}m.$$ The coordination number of each ion in $$AB$$ is
A
4
B
8
C
2
D
6
Answer :
6
159. The term that corrects for the attractive forces present in a real gas in the van der Waals equation is
A
$$nb$$
B
$$\frac{{a{n^2}}}{{{V^2}}}$$
C
$$ - \frac{{a{n^2}}}{{{V^2}}}$$
D
$$ - nb$$
Answer :
$$\frac{{a{n^2}}}{{{V^2}}}$$
160. The pressure of a 1 : 4 mixture of dihydrogen and dioxygen enclosed in a vessel is one atmosphere. What would be the partial pressure of dioxygen ?
A
$$0.8 \times {10^5}\,atm$$
B
$$0.008\,N\,{m^{ - 2}}$$
C
$$8 \times {10^4}N\,{m^{ - 2}}$$
D
$$0.25\,atm$$
Answer :
$$8 \times {10^4}N\,{m^{ - 2}}$$