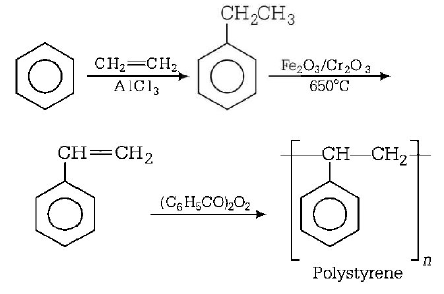
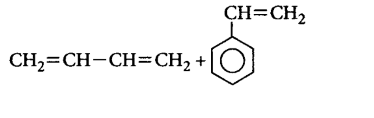
31. Which one of the following is a chain growth polymer ?
A
Starch
B
Nucleic acid
C
Polystyrene
D
Protein
Answer :
Polystyrene
32. Which one of the following is an example of a thermosetting polymer ?
A


B
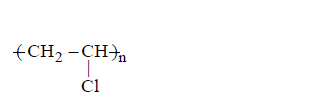
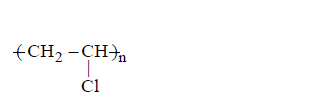
C


D


Answer :


33. Which of the following is fully fluorinated polymer?
A
$$PVC$$
B
Thiokol
C
Teflon
D
Neoprene
Answer :
Teflon
34. The monomers used in addition polymerisation through free radical should be very pure because
A
the traces of impurities act like inhibitors resulting in short chain polymers
B
the impurities result in formation of different products
C
the polymer formed is impure
D
catalyst does not function in presence of impurities
Answer :
the traces of impurities act like inhibitors resulting in short chain polymers
35. In which of the following polymers, empirical formula resembles with monomer ?
A
Bakelite
B
Teflon
C
Nylon - 6, 6
D
Dacron
Answer :
Teflon
36. Nylon is an example of
A
polyester
B
polysaccharide
C
polyamide
D
polythene
Answer :
polyamide
37. Which of the following is not an example of rubber?
A
Polychloroprene
B
Buna-$$N$$
C
Butadiene-styrene copolymer
D
Polyacrylonitrile
Answer :
Polyacrylonitrile
38. Which one of the following statement is not true ?
A
In vulcanization the formation of sulphur bridges between different chains make rubber harder and stronger.
B
Natural rubber has the $$trans$$ - configuration at every double bond
C
$$Buna-S$$ is a copolymer of butadiene and styrene
D
Natural rubber is a 1, 4 - polymer of isoprene
Answer :
Natural rubber has the $$trans$$ - configuration at every double bond
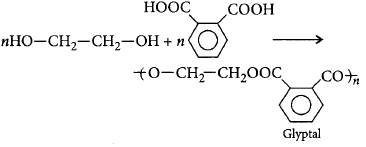
40. In which of the following polymers ethylene glycol is one of the monomer units?
A


B
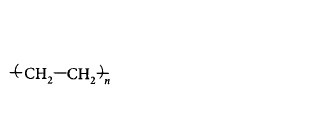
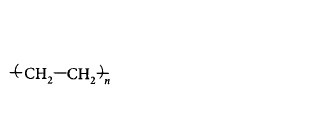
C
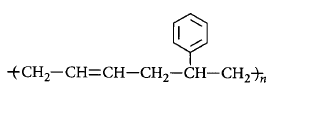

D


Answer :