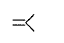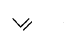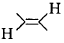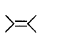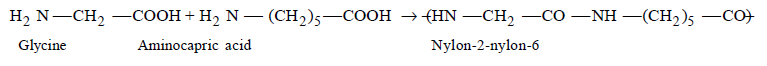151. Glyptal polymer is obtained by the following monomers,
A
malonic acid + ethylene glycol
B
phthalic acid + ethylene glycol
C
maleic acid + formaldehyde
D
acetic acid + phenol
Answer :
phthalic acid + ethylene glycol
152. Which of the following alkenes is most reactive towards cationic polymerisation?
A
$$C{H_2} = CHC{H_3}$$
B
$$C{H_2} = CHCl$$
C
$$C{H_2} = CH{C_6}{H_5}$$
D
$$C{H_2} = CHCOOC{H_3}$$
Answer :
$$C{H_2} = CH{C_6}{H_5}$$
153. Synthetic polymer bakelite can be prepared from following compounds
A
Styrene and vinyl chloride
B
Acrylonitrile and vinyl chloride
C
Adipic acid and ethylene glycol
D
Phenol and formaldehyde
Answer :
Phenol and formaldehyde
155. Mark the incorrect use of the polymer.
A
High density - Buckets, pipes, polythene
B
Nylon 6, 6 - Ropes, bristles for brushes
C
Orlon - Synthetic wool, carpets
D
Glyptal - Electrical switches, combs
Answer :
Glyptal - Electrical switches, combs
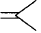
156. Biodegradable polymer which can be produced from glycine and aminocaproic acid is :
A
$$PHBV$$
B
$$Buna - N$$
C
$${\text{Nylon 6, 6}}$$
D
$${\text{Nylon 2 - nylon 6}}$$
Answer :
$${\text{Nylon 2 - nylon 6}}$$
157. Polymer formation from monomers starts by
A
condensation or addition reaction between monomers
B
coordinate reaction between monomers
C
conversion of monomer to monomer ions
D
hydrolysis of monomers.
Answer :
condensation or addition reaction between monomers
158. Given the polymers (i) Nylon - 6, 6; (ii) Buna - $$S;$$ (iii) Polythene. Arrange these in increasing order of their inter-molecular forces ( lower to higher )
A
(i) > (ii) > (iii)
B
(ii) > (iii) > (i)
C
(ii) < (iii) < (i)
D
(iii) < (i) < (ii)
Answer :
(ii) < (iii) < (i)
159. Which of the following statements is not correct for fibres ?
A
Fibres possess high tensile strength and high modulus.
B
Fibres impart crystalline nature.
C
Characteristic features of fibres are due to strong intermolecular forces like hydrogen bonding.
D
All are correct.
Answer :
All are correct.
160. When condensation product of hexamethylene-diamine and adipic acid is heated to $$525\,K$$ in an atmosphere of nitrogen for about 4 - 5 hours, the product obtained is
A
solid polymer of nylon 6, 6
B
liquid polymer of nylon 6, 6
C
gaseous polymer of nylon 6, 6
D
liquid polymer of nylon 6
Answer :
liquid polymer of nylon 6, 6
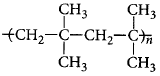 is a polymer having monomer units ________.
is a polymer having monomer units ________.