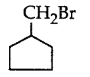
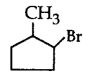
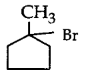
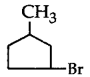
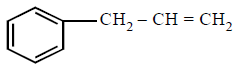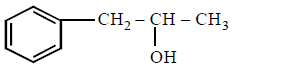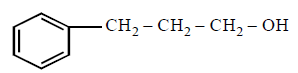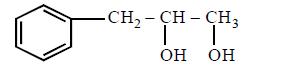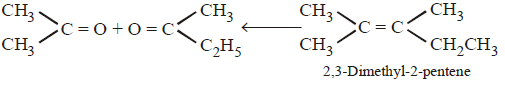281. The compound that will react most readily with gaseous bromine has the formula
A
$${C_3}{H_6}$$
B
$${C_2}{H_2}$$
C
$${C_4}{H_{10}}$$
D
$${C_2}{H_4}$$
Answer :
$${C_4}{H_{10}}$$
282.
Choose the correct reagents used in the conversion.
\[C{{H}_{2}}=C{{H}_{2}}\xrightarrow{\left( p \right)}\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\, \\
Br\,\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{C{{H}_{2}}}}\,-\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\, \\
Br\,\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{C{{H}_{2}}}}\,\] \[\xrightarrow{\left( q \right)}C{{H}_{2}}=CHBr\xrightarrow{\left( r \right)}\] \[CH\equiv CH\xrightarrow{\left( s \right)}\] 
$$p$$
$$q$$
$$r$$
$$s$$
(a)
$$B{r_2}$$
$${\text{alc}}.\,KOH$$
$$NaOH$$
$$A{l_2}{O_3}$$
(b)
$$HBr$$
$${\text{alc}}.\,KOH$$
$$Ca{C_2}$$
$$KMn{O_4}$$
(C)
$$HBr$$
$${\text{alc}}.\,KOH$$
$$NaN{H_2}$$
red hot iron tube
(d)
$$B{r_2}$$
$${\text{alc}}.\,KOH$$
$$NaN{H_2}$$
red hot iron tube
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(d)
284. When cyclohexane is poured on water, it floats, because :
A
cyclohexane is in ‘boat’ form
B
cyclohexane is in ‘chair’ form
C
cyclohexane is in ‘crown’ form
D
cyclohexane is less dense than water
Answer :
cyclohexane is less dense than water
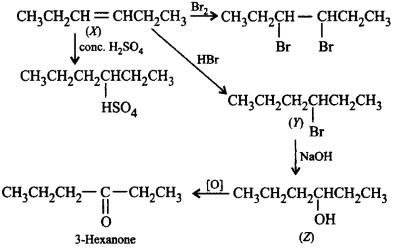
285.
A compound $$X$$ decolourises $$B{r_2}$$ water and reacts slowly with conc. $${H_2}S{O_4}$$ to givean addition product. $$X$$ reacts with $$HBr$$ to form $$Y.$$ $$Y$$ reacts with $$NaOH$$ to form $$Z.$$ On oxidation $$Z$$ gives hexan-3-one. $$X, Y$$ and $$Z$$ in the reactions are
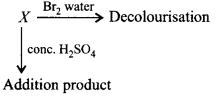
\[X\xrightarrow{HBr}Y\xrightarrow{NaOH}Z\xrightarrow{\left[ O \right]}\] \[\underset{\begin{align}
& \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\parallel \\
& \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,O \\
& \,\,\,\,\,\,\,\,\,\,\,\text{Hexan-3-one} \\
& \\
\end{align}}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}-C-C{{H}_{2}}C{{H}_{3}}}}\,\]
A
$$X = C{H_3}C{H_2}CH = CHC{H_3},$$ $$Y = C{H_3}C{H_2}CH\left( {Br} \right)CH\left( {Br} \right)C{H_2}C{H_3},$$ $$Z = C{H_3}C{H_2}C{H_3}$$
B
$$X = C{H_3}CH = CHC{H_3},$$ $$Y = C{H_3}CH\left( {Br} \right)CH\left( {Br} \right)C{H_3},$$ $$Z = C{H_3}C{H_2}C{H_2}OH$$
C
$$X = C{H_3}C{H_2}CH = CHC{H_2}C{H_3},$$ \[Y=C{{H}_{3}}C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
Br\,\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}C{{H}_{2}}C{{H}_{3}},\] \[Z=C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
OH\,\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}C{{H}_{3}}\]
D
$$X = C{H_3}C{H_2}C{H_2}CH = CHC{H_3},$$ $$Y = C{H_3}C{H_2}C{H_2}C{H_2}C{H_2}C{H_2}Br,$$ $$Z = C{H_3}C{H_2}C{H_2}C{H_2}OH$$
Answer :
$$X = C{H_3}C{H_2}CH = CHC{H_2}C{H_3},$$ \[Y=C{{H}_{3}}C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
Br\,\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}C{{H}_{2}}C{{H}_{3}},\] \[Z=C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
OH\,\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}C{{H}_{3}}\]
286. Which of the following statements is true?
A
Soda lime is a mixture of sodium hydroxide and potassium hydroxide.
B
Methane can be prepared by Wurtz reaction.
C
In alkanes all carbon atoms are $$s{p^3}$$ hybridised.
D
$$neo$$ - Pentane yields three different monochloro derivatives.
Answer :
In alkanes all carbon atoms are $$s{p^3}$$ hybridised.
287. What is the order of reactivity of hydrogen atoms attached to carbon atom in an alkene?
A
$${3^ \circ } > {1^ \circ } > {2^ \circ }$$
B
$${2^ \circ } > {1^ \circ } > {3^ \circ }$$
C
$${3^ \circ } > {2^ \circ } > {1^ \circ }$$
D
$${1^ \circ } > {2^ \circ } > {3^ \circ }$$
Answer :
$${3^ \circ } > {2^ \circ } > {1^ \circ }$$
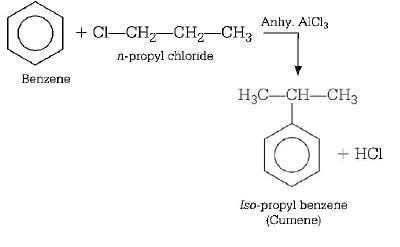
289. Benzene reacts with $$n$$ -propyl chloride in the presence of anhydrous $$AlC{l_3}$$ to give
A
3 - propyl - 1 - chlorobenzene
B
$$n$$ - propyl benzene
C
no reaction
D
$$iso$$ - propyl benzene
Answer :
$$iso$$ - propyl benzene
290.
An alkene having molecular formula $${C_7}{H_{14}}$$ was subjected to ozonolysis in the presence of zinc dust. An equimolar amount of the following two compounds was obtained
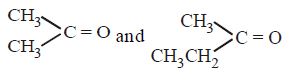
The $$IUPAC$$ name of the alkene is
A
3, 4 - dimethyl - 3 - pentene
B
3, 4 - dimethyl - 2 - pentene
C
2, 3 - dimethyl - 3 - pentene
D
2, 3 - dimethyl - 2 - pentene
Answer :
2, 3 - dimethyl - 2 - pentene