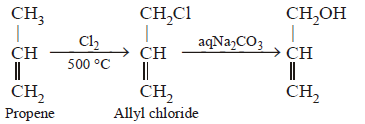
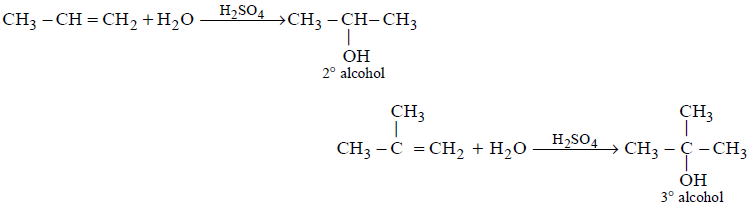261. Which of the following compounds will not undergoes Friedel-Craft’s reaction easily?
A
Cumene
B
Xylene
C
Nitrobenzene
D
Toluene
Answer :
Nitrobenzene
262. In the presence of peroxide, $$HCl$$ and $$HI$$ do not give anti-Markownikoff’s addition of alkenes because :
A
One of the steps is endothermic in $$HCl$$ and $$HI$$
B
Both $$HCl$$ and $$HI$$ are strong acids
C
$$HCl$$ is oxidizing and the $$HI$$ is reducing
D
All the steps are exothermic is $$HCl$$ and $$HI$$
Answer :
One of the steps is endothermic in $$HCl$$ and $$HI$$
263. The reaction of propene with $$HOCl$$ proceeds via the addition of
A
$${H^ + }$$ in the first step
B
$$C{l^ + }$$ in the first step
C
$$O{H^ - }$$ in the first step
D
$$C{l^ + }$$ and $$O{H^ - }$$ in a single step
Answer :
$$C{l^ + }$$ in the first step
264.
Identify $$A, B$$ and $$C$$ in following reactions :

A
$$A = Ni,\,B = {H_2}O{\text{ (liquid),}}\,C = {H_2}O$$
B
$$A = Zn,\,B = {H_2}O{\text{ (steam),}}\,C = LiAl{H_4}$$
C
$$A = Mg,\,B = {H_2}O\,{\text{(liquid),}}\,C = HCl$$
D
$$A = Sn,\,B = {H_2}O\,({\text{boiling),}}\,C = SnC{l_2}$$
Answer :
$$A = Zn,\,B = {H_2}O{\text{ (steam),}}\,C = LiAl{H_4}$$
265.
Identify the product, P in the following reaction :
$$C{H_3} - CH = C{H_2} + NOCl \to P$$
A
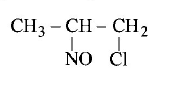
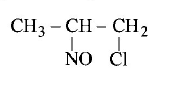
B


C
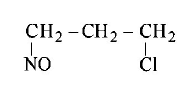
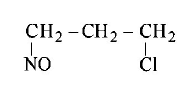
D
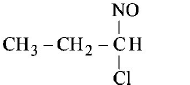
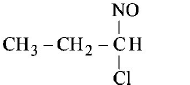
Answer :


266. In halogenation of aromatic hydrocarbon, a halogen carrier is used which is generally a Lewis acid. The main function of this reagent is to generate the specie
A
$$X$$
B
$${X^ - }$$
C
$${X^ + }$$
D
$$\mathop X\limits^{\,\, \bullet } $$
Answer :
$${X^ + }$$
267.
Consider the following sequence of reactions
\[C{{H}_{3}}CH=C{{H}_{2}}\xrightarrow[700K]{C{{l}_{2}}}A\xrightarrow[420K,12\,atm]{N{{a}_{2}}C{{O}_{3}}}B\]
Compound $$'B'$$ is
A


B


C


D


Answer :


268.
 \[+\,Cl-C{{H}_{2}}C{{H}_{2}}-C{{H}_{3}}\xrightarrow{AlC{{l}_{3}}}P\] \[\xrightarrow[\left( \text{ii} \right){{H}_{3}}{{O}^{+}}]{\left( \text{i} \right)\frac{{{O}_{2}}}{\Delta }}Q+\text{phenol}\]
\[+\,Cl-C{{H}_{2}}C{{H}_{2}}-C{{H}_{3}}\xrightarrow{AlC{{l}_{3}}}P\] \[\xrightarrow[\left( \text{ii} \right){{H}_{3}}{{O}^{+}}]{\left( \text{i} \right)\frac{{{O}_{2}}}{\Delta }}Q+\text{phenol}\]
The major products $$P$$ and $$Q$$ are
A


B


C


D


Answer :


269. Acid catalyzed hydration of alkenes except ethene leads to the formation of
A
mixture of secondary and tertiary alcohols
B
mixture of primary and secondary alcohols
C
secondary or tertiary alcohol
D
primary alcohol
Answer :
secondary or tertiary alcohol
270. An inhibitor is described as,
A
a substance that slows down or stops a reaction
B
a substance which inhibits the properties of a catalyst
C
a substance formed during the reaction and does not participate in the reaction
D
a substance which prevents formation of products in a reaction being most reactive
Answer :
a substance that slows down or stops a reaction