251.
Which one of the following alkenes will react faster with $${H_2}$$ under catalytic hydrogenation conditions ?
( $$R=$$ alkyl substituent )
A
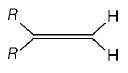

B


C


D


Answer :


252.
Which of the following compounds are antiaromatic
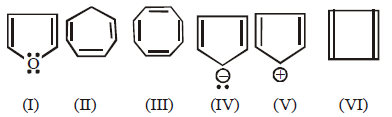
A
(I) and (V)
B
(II) and (V)
C
(I) and (IV)
D
(V) and (VI)
Answer :
(V) and (VI)
253. Acetylenic hydrogens are acidic because
A
sigma electron density of $$C-H$$ bond in acetylene is nearer to carbon which has 50% $$s$$ - character
B
acetylene has only one hydrogen on each carbon
C
acetylene contains least number of hydrogens among the possible hydrocarbons having two carbons
D
acetylene belong to the class of alkynes with molecular formula $${C_n}{H_{2n - 2}}$$
Answer :
sigma electron density of $$C-H$$ bond in acetylene is nearer to carbon which has 50% $$s$$ - character
254.

Which of the following products cannot be obtained in ozonolysis of $$o$$ - xylene ?
A


B
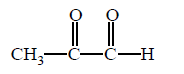

C
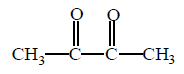

D
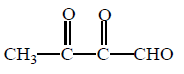
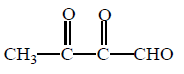
Answer :
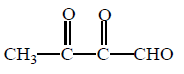
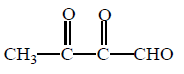
255. The reaction in terms of intermediates and type of reaction is given below. Mark the incorrect option.
A
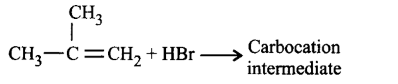

B


C
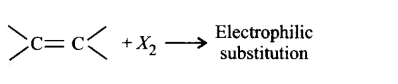

D
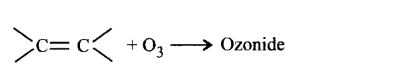

Answer :


256. $$Anti$$ -Markovnikoff addition of $$HBr$$ is not observed in :
A
propene
B
1 - butene
C
but - 2 - ene
D
pent - 2 - ene
Answer :
but - 2 - ene
257.
Predict the product $$C$$ obtained in the following reaction of butyne-1.
\[C{{H}_{3}}C{{H}_{2}}-C\equiv CH+HCl\to B\xrightarrow{HI}C\]
A


B


C


D


Answer :


258. The trans-alkenes are formed by the reduction of alkynes with :
A
$${H_2} - Pd/C,\,BaS{O_4}$$
B
$$NaB{H_4}$$
C
$$Na/liq.\,N{H_3}$$
D
$$Sn - HCl$$
Answer :
$$Na/liq.\,N{H_3}$$
259.
The correct IUPAC name of the following alkane is

A
3, 6-diethyl-2-methyloctane
B
5-isopropyl-3-ethyloctane
C
3-ethyl-5-isopropyloctane
D
3-isopropyl-6-ethyloctane
Answer :
3, 6-diethyl-2-methyloctane
260.
Which of the following types of reaction occur when a reactant has got a double bond ?
(i) Addition
(ii) Photolysis
(iii) Nucleophilic substitution
(iv) Polymerization
A
(i) and (iv)
B
(i) and (ii)
C
(i), (ii) and (iv)
D
(i), (ii), (iii) and (iv)
Answer :
(i) and (iv)


 ( $$o$$ - xylene ) which on ozonolysis give A, B and C.
( $$o$$ - xylene ) which on ozonolysis give A, B and C. 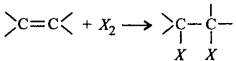 is an example of electrophilic addition reaction.
is an example of electrophilic addition reaction. 

