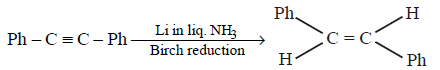241. In the free radical chlorination of methane, the chain initiating step involves the formation of
A
chlorine free radical
B
hydrogen chloride
C
methyl radical
D
chloromethyl radical
Answer :
chlorine free radical
242. The correct order of reactivity towards electrophilic substitution is
A
benzene > phenol > benzoic acid > chlorobenzene
B
phenol > benzene > chlorobenzene > benzoic acid
C
chlorobenzene > benzoicacid > phenol > benzene
D
benzoicacid > chlorobenzene > benzene > phenol
Answer :
phenol > benzene > chlorobenzene > benzoic acid
243.

Identify the position where electrophilic aromatic substitution $$(EAS)$$ is most favourable.
A
$$A$$
B
$$B$$
C
$$C$$
D
$$A\,\,{\text{and}}\,\,C$$
Answer :
$$B$$
244. Which of the following decolourises alkaline $$KMn{O_4}$$
A
$${C_3}{H_8}$$
B
$${C_2}{H_4}$$
C
$$C{H_4}$$
D
$$CC{l_4}$$
Answer :
$${C_2}{H_4}$$
245. An alkene $$X$$ is obtained by dehydration of an alcohol $$Y.$$ $$X$$ on ozonolysis gives two molecules of ethanal for every molecule of alkene. $$X$$ and $$Y$$ are
A
$$X = 3{\text{ - hexene, }}Y = 3{\text{ - hexanol}}$$
B
$$X = 2{\text{ - butene, }}Y = 2{\text{ - butanol }}$$
C
$$X = {\text{1 - butene, }}Y = 1{\text{ - butanol }}$$
D
$$X = {\text{1 - hexane, }}Y = 1{\text{ - hexanol}}$$
Answer :
$$X = 2{\text{ - butene, }}Y = 2{\text{ - butanol }}$$
246.

\[B{{r}^{\centerdot }}\] will abstract which of the hydrogen most readily ?
A
a
B
b
C
c
D
d
Answer :
a
248. Pentene-1 with $$HCl$$ gives
A
3-chloropentane
B
2-chloropentane
C
1, 2-dichloropentane
D
1-chloropentane
Answer :
2-chloropentane
249.
The following reaction is known as
\[{{C}_{6}}{{H}_{6}}+C{{H}_{3}}Cl\xrightarrow[\text{(anhy}\text{.})]{AlC{{l}_{3}}}\] $${C_6}{H_5}C{H_3} + HCl$$
A
Wurtz-Fittig reaction
B
Friedel-Crafts reaction
C
Rosenmund reaction
D
Sandmeyer reaction
Answer :
Friedel-Crafts reaction
250.
The reagent needed for converting

A
$${\text{Cat}}.{\text{ Hydrogenation}}$$
B
$${H_2}/{\text{Lindlar Cat}}{\text{.}}$$
C
$$Li/N{H_3}$$
D
$$LiAl{H_4}$$
Answer :
$$Li/N{H_3}$$

 ( $$+ M$$ group ) ( More activating group )
( $$+ M$$ group ) ( More activating group )  ( \[{{3}^{\circ }}\] free-radical ) is most stable.
( \[{{3}^{\circ }}\] free-radical ) is most stable.