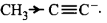231. Acidic hydrogen is present in :
A
ethyne
B
ethene
C
benzene
D
ethane
Answer :
ethyne
232.
Identify $$(A), (B)$$ and $$(C).$$
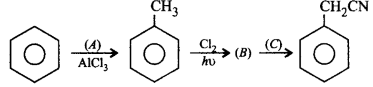
A
$$A \to C{H_3}COCl,\,B \to {C_6}{H_5}Cl,$$ $$C \to NaCN$$
B
$$A \to C{H_3}Cl,\,B \to {C_6}{H_5}C{H_2}Cl,$$ $$C \to KCN$$
C
$$A \to C{H_4},\,B \to {C_6}{H_5}C{H_2}Cl,$$ $$C \to AgCN$$
D
$$A \to C{H_3}Cl,\,B \to {C_6}{H_5}COCl,$$ $$C \to KCN$$
Answer :
$$A \to C{H_3}Cl,\,B \to {C_6}{H_5}C{H_2}Cl,$$ $$C \to KCN$$
233. The compound with the highest boiling point is
A
$$n $$ - hexane
B
$$n $$ - pentane
C
2, 2 - dimethylpropane
D
2 - methylbutane
Answer :
$$n $$ - hexane
234. In allene $$\left( {{C_3}{H_4}} \right)$$ , the type$$(s)$$ of hybridisation of the carbon atoms is (are) :
A
$$sp\,{\text{and}}\,s{p^3}$$
B
$$sp\,{\text{and}}\,s{p^2}$$
C
$${\text{only}}\,s{p^3}$$
D
$$s{p^2}\,{\text{and}}\,s{p^3}$$
Answer :
$$sp\,{\text{and}}\,s{p^2}$$
235. In Friedel-Craft’s alkylation, besides $$AlC{l_3}$$ the other reactants are
A
$${C_6}{H_6} + N{H_2}$$
B
$${C_6}{H_6} + C{H_4}$$
C
$${C_6}{H_6} + C{H_3}Cl$$
D
$${C_6}{H_6} + C{H_3}COCl$$
Answer :
$${C_6}{H_6} + C{H_3}Cl$$
236.
Arrange the following carbanions in order of their decreasing stability.
$$\eqalign{
& \left( {\text{i}} \right){H_3}C - C \equiv {C^ - } \cr
& \left( {{\text{ii}}} \right)H - C \equiv {C^ - } \cr
& \left( {{\text{iii}}} \right){H_3}C - CH_2^ - \cr} $$
A
(i) > (ii) > (iii)
B
(ii) > (i) > (iii)
C
(iii) > (ii) > (i)
D
(iii) > (i) > (ii)
Answer :
(ii) > (i) > (iii)
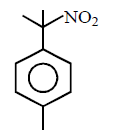
238. In the reaction with $$HCl,$$ an alkene reacts in accordance with the Markownikoff’s rule, to give a product $$1-chloro-1-methylcyclohexane.$$ The possible alkane is
A


B


C
D
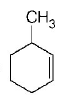

Answer :
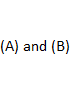
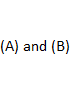
239.
The hottest region of Bunsen flame shown in the figure
below is :
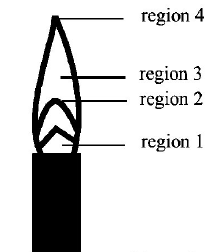
A
region 3
B
region 4
C
region 1
D
region 2
Answer :
region 2
240. The correct statement regarding the comparison of staggered and eclipsed conformations of ethane, is
A
The eclipsed conformation of ethane is more stable than staggered conformation, because
eclipsed conformation has no torsional strain
B
The eclipsed conformation of ethane is more stable than staggered conformation even
though the eclipsed conformation has torsional strain
C
The staggered conformation of ethane is more stable than eclipsed conformation, because
staggered conformation has no torsional strain
D
The staggered conformation of ethane is less stable than eclipsed conformation, because
staggered conformation has torsional strain
Answer :
The staggered conformation of ethane is more stable than eclipsed conformation, because
staggered conformation has no torsional strain