201. Arrange the halogens $${F_2},C{l_2},B{r_2},{I_2},$$ in order of their increasing reactivity with alkanes.
A
$${I_2} < B{r_2} < C{l_2} < {F_2}$$
B
$$B{r_2} < C{l_2} < {F_2} < {I_2}$$
C
$${F_2} < C{l_2} < B{r_2} < {I_2}$$
D
$$B{r_2} < {I_2} < C{l_2} < {F_2}$$
Answer :
$${I_2} < B{r_2} < C{l_2} < {F_2}$$
202.
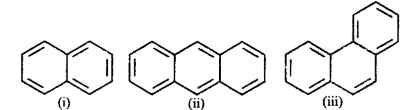
Identify the polynuclear aromatic compound which is aromatic.
A
(i) and (ii)
B
(ii) and (iii)
C
(i), (ii) and (iii)
D
(i) and (iii)
Answer :
(i), (ii) and (iii)
203. Identify the reagent which can easily distinguish between 1-butyne and 2-butyne.
A
Bromine water
B
Baeyer's reagent
C
Dilute $${H_2}S{O_4} + HgS{O_4}$$
D
Ammoniacal $$C{u_2}C{l_2}$$
Answer :
Ammoniacal $$C{u_2}C{l_2}$$
204. Hydrocarbon which is liquid at room temperature is
A
pentane
B
butane
C
propane
D
ethane
Answer :
pentane
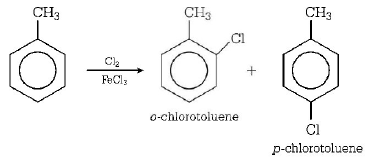
205. The reaction of toluene with $$C{l_2}$$ in the presence of $$FeC{l_3}$$ gives $$'X'$$ and reaction in presence of light gives $$'Y’.$$ Thus, $$'X’$$ and $$'Y’$$ are
A
$$X=$$ benzal chloride, $$Y = $$ $$o$$ - chlorotoluene
B
$$X =$$ $$m$$ - chlorotoluene, $$Y =$$ $$p$$ - chlorotoluene
C
$$X =o$$ and $$p$$ - chlorotoluene, $$Y =$$ trichloromethyl benzene
D
$$X =$$ benzyl chloride, $$Y =$$ $$m$$ - chlorotoluene
Answer :
$$X =o$$ and $$p$$ - chlorotoluene, $$Y =$$ trichloromethyl benzene
206. The shortest $$C-C$$ bond distance is found in
A
diamond
B
ethane
C
benzene
D
acetylene
Answer :
acetylene
207.
Consider the following reaction
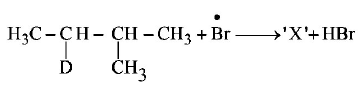
Identify the structure of the major product $$'X’$$
A


B
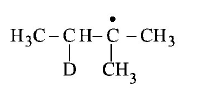
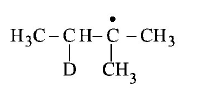
C


D


Answer :
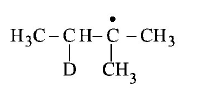
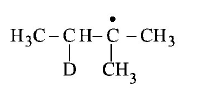
208. Chlorination of methane does not occur in dark because
A
methane can form free radicals in presence of sunlight only
B
to get chlorine free radicals from $$C{l_2}$$ molecules energy is required
C
substitution reaction can take place only in sunlight and not in dark
D
termination step cannot take place in dark. It requires sunlight
Answer :
to get chlorine free radicals from $$C{l_2}$$ molecules energy is required
210. An unknown compound $$A$$ has a molecular formula $${C_4}{H_6},$$ when $$A$$ is treated with an excess of $$B{r_2},$$ a new substance $$B$$ with formula $${C_4}{H_6}B{r_2}$$ is formed. $$A$$ forms a white precipitate with ammonical silver nitrate solution. $$A$$ may be
A
Butyne-1
B
Butyne-2
C
Butene-1
D
Butene-2
Answer :
Butyne-1
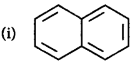 $$4n + 2 = 10 \Rightarrow n = 2$$
$$4n + 2 = 10 \Rightarrow n = 2$$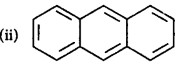 $$4n + 2 = 14 \Rightarrow n = 3$$
$$4n + 2 = 14 \Rightarrow n = 3$$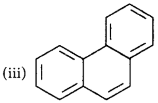 $$4n + 2 = 14 \Rightarrow n = 3$$
$$4n + 2 = 14 \Rightarrow n = 3$$