621. The first member of the $$p$$ - block elements differs from the remaining members of their corresponding groups due to
A
small size and absence of $$d$$ - orbitals
B
diagonal relationship with other elements
C
difference in ability to form double and triple bonds
D
high ionisation enthalpy
Answer :
small size and absence of $$d$$ - orbitals
622. The correct order of reactivity of halogens with alkalies is
A
$$F > Cl > Br > I$$
B
$$F < Cl > Br < I$$
C
$$F < Cl < Br < I$$
D
$$F < Cl < Br > I$$
Answer :
$$F > Cl > Br > I$$
623. Which of the following oxides will be the least acidic?
A
$$A{s_4}{O_6}$$
B
$$A{s_4}{O_{10}}$$
C
$${P_4}{O_{10}}$$
D
$${P_4}{O_6}$$
Answer :
$$A{s_4}{O_6}$$
624. Graphite is a soft solid lubricant extremely difficult to melt. The reason for this anomalous behaviour is that graphite
A
is an allotropic form of diamond
B
has molecules of variable molecular masses like
polymers
C
has carbon atoms arranged in large plates of rings of
strongly bound carbon atoms with weak interplate
bonds
D
is a non-crystalline substance
Answer :
has carbon atoms arranged in large plates of rings of
strongly bound carbon atoms with weak interplate
bonds
625. Which of the following group - 14 elements is a radioactive element?
A
Flerovium
B
Germanium
C
Nihonium
D
Gallium
Answer :
Flerovium
626. Glass and cement are two important examples of
A
man-made silicates
B
silicones
C
zeolites
D
organic polymers
Answer :
man-made silicates
627. Which among the following statements is incorrect?
A
$$Xe{F_4}$$ and $$Sb{F_5}$$ combine to form salt.
B
$$He$$ and $$Ne$$ do not form clathrates.
C
$$He$$ has highest boiling point in its group.
D
$$He$$ diffuses through rubber and polyvinyl chloride.
Answer :
$$He$$ has highest boiling point in its group.
628.
Which of the following statements are correct ?
(i) Aluminium forms $${\left[ {Al{F_6}} \right]^{3 - }}ion$$ while boron forms only $${\left[ {B{F_4}} \right]^ - }\,ion$$ due to presence of $$d$$ - orbitals in aluminium.
(ii) The first member of a group differs from the heavier members in its ability to form $$p\pi {\text{ - }}p\pi $$ multiple bonds to itself and to other second row elements. While heavier member forms $$d\pi {\text{ - }}p\pi $$ bonds.
(iii) $$d$$ - orbitals contribute more to the overall stability of molecules than $$p\pi {\text{ - }}p\pi $$ bonding of second row elements.
A
(i) (ii) (iii)
B
(i) (iii)
C
(i) (ii)
D
(ii) (iii)
Answer :
(i) (ii)
629.
An inorganic compound $$'A'$$ shows the following reactions :
(i) It is white solid, exists as dimer and fumes in wet air.
(ii) It sublimes at $$180{\,^ \circ }C$$ and forms monomer if heated to $$400{\,^ \circ }C.$$
(iii) Its aqueous solution turns blue litmus to red and gives a white precipitate with $$AgN{O_3}$$ solution, which is soluble in $$N{H_4}OH.$$
(iv) Addition of $$N{H_4}OH$$ and $$NaOH$$ separately to the solution of $$'A'$$ gives a gelatinous precipitate which in however soluble in excess of $$NaOH.$$
The compound $$'A'$$ is
A
$$Al{\left( {OH} \right)_3}$$
B
$$A{l_2}C{l_6}$$
C
$$A{l_2}{O_3}$$
D
$$A{l_2}{\left( {S{O_4}} \right)_3}$$
Answer :
$$A{l_2}C{l_6}$$
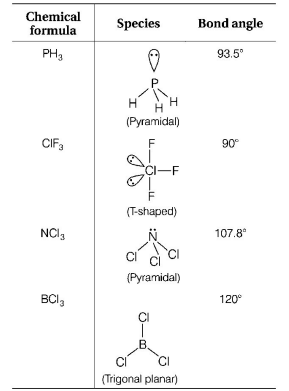
630. The species, having bond angles of $${120^ \circ }$$ is
A
$$P{H_3}$$
B
$$Cl{F_3}$$
C
$$NC{l_3}$$
D
$$BC{l_3}$$
Answer :
$$BC{l_3}$$