581. Aluminium chloride exists as dimer, $$A{l_2}C{l_6}$$ in solid state as well as in solution of non-polar solvents such as benzene. When dissolved in water, it gives
A
$${\left[ {Al{{\left( {OH} \right)}_6}} \right]^{3 - }} + 3HCl$$
B
$${\left[ {Al{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }} + 3C{l^ - }$$
C
$$A{l^{3 + }} + 3C{l^ - }$$
D
$$A{l_2}{O_3} + 6HCl$$
Answer :
$${\left[ {Al{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }} + 3C{l^ - }$$
582. Which out of the following compounds does not exist ?
A
$$B{F_3}$$
B
$$TlC{l_3}$$
C
$$TlC{l_5}$$
D
$${\text{Both (B) and (C)}}$$
Answer :
$$TlC{l_5}$$
583.
Which of the following is correct representation of the reaction when $$B{F_3}$$ reacts with ammonia?
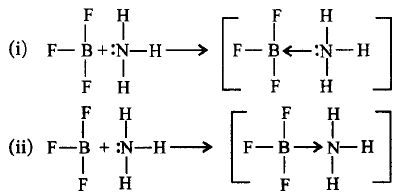
A
(i) is incorrect and (ii) is correct.
B
(i) is correct and (ii) is incorrect.
C
Both (i) and (ii) are correct.
D
Both (i) and (ii) are incorrect.
Answer :
(i) is correct and (ii) is incorrect.
584. Density of nitrogen gas prepared from air is slightly greater than that of nitrogen prepared by chemical reaction from a compound of nitrogen due to the presence of
A
argon
B
carbon dioxide
C
some $${N_3}$$ molecules analogous to $${O_3}.$$
D
greater amount of $${N_2}$$ molecules derived from $$N{\text{ - }}15$$ isotope.
Answer :
argon
585. Which of the following form of the sulphur shows paramagnetic behaviour ?
A
$${S_8}$$
B
$${S_6}$$
C
$${S_2}$$
D
$${\text{All of these}}$$
Answer :
$${S_2}$$
586. $${H_2}S{O_4}$$ and $${H_2}S{O_3}$$ can be distinguished by the addition of
A
magnesium powder
B
$$NaHS{O_4}$$ solution
C
$$FeC{l_3}$$ solution
D
litmus solution
Answer :
$$FeC{l_3}$$ solution
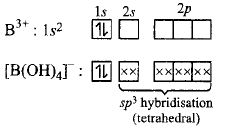
587. The geometry of a complex species can be understood from the knowledge of type of hybridisation of orbitals of central atom. The hybridisation of orbitals of central atom in $${\left[ {B{{\left( {OH} \right)}_4}} \right]^ - }$$ and the geometry of the complex are respectively
A
$$s{p^3},$$ tetrahedral
B
$$s{p^3},$$ square planar
C
$$s{p^3}{d^2},$$ octahedral
D
$$ds{p^2},$$ square planar
Answer :
$$s{p^3},$$ tetrahedral
588. Hot conc. $${H_2}S{O_4}$$ acts as moderately strong oxidising agent. It oxidises both metals and non-metals. Which of the following elements is oxidised by conc. $${H_2}S{O_4}$$ into two gaseous products?
A
$$Cu$$
B
$$S$$
C
$$C$$
D
$$Zn$$
Answer :
$$C$$
589. Among the following oxoacids, the correct decreasing order of acid strength is:
A
$$HOCl > HCl{O_2} > HCl{O_3} > HCl{O_4}$$
B
$$HCl{O_4} > HOCl > HCl{O_2} > HCl{O_3}$$
C
$$HCl{O_4} > HCl{O_3} > HCl{O_2} > HOCl$$
D
$$HCl{O_2} > HCl{O_4} > HCl{O_3} > HOCl$$
Answer :
$$HCl{O_4} > HCl{O_3} > HCl{O_2} > HOCl$$
590. The most commonly used reducing agent is
A
$$AlC{l_3}$$
B
$$PbC{l_2}$$
C
$$SnC{l_4}$$
D
$$SnC{l_2}$$
Answer :
$$SnC{l_2}$$