551. It is because of inability of $$n{s^2}$$ electrons of the valence shell to participate in bonding that
A
$$S{n^{2 + }}$$ is reducing while $$P{b^{4 + }}$$ is oxidising
B
$$S{n^{2 + }}$$ is oxidising while $$P{b^{4 + }}$$ is reducing
C
$$S{n^{2 + }}$$ and $$P{b^{2 + }}$$ are both oxidising and reducing
D
$$S{n^{4 + }}$$ is reducing while $$P{b^{4 + }}$$ is oxidising
Answer :
$$S{n^{4 + }}$$ is reducing while $$P{b^{4 + }}$$ is oxidising
552. The correct order of the oxidation states of nitrogen in $$NO,{N_2}O,N{O_2}\,{\text{and}}\,{N_2}{O_3}$$ is:
A
$$N{O_2} < NO < {N_2}{O_3} < {N_2}O$$
B
$$N{O_2} < {N_2}{O_3} < NO < {N_2}O$$
C
$${N_2}O < \,{N_2}{O_3} < NO < N{O_2}$$
D
$${N_2}O < \,NO < {N_2}{O_3} < N{O_2}$$
Answer :
$${N_2}O < \,NO < {N_2}{O_3} < N{O_2}$$
553.
Fill in the blanks by choosing an appropriate option. $$\underline {\left( {\text{i}} \right)} $$ is a synthetic radioactive element of group 15 having electronic configuration $$\underline {\left( {{\text{ii}}} \right)} .$$
(i)
(ii)
(a)
$$_{115}MC$$
$$\left[ {Rn} \right]5{f^{14}}6{d^{10}}7{s^2}7{p^3}$$
(b)
$$_{115}MC$$
$$\left[ {Xe} \right]5{f^{14}}6{d^{10}}7{s^2}7{p^3}$$
(c)
$$_{116}Lv$$
$$\left[ {Rn} \right]5{f^{14}}6{d^{10}}7{s^2}7{p^4}$$
(d)
$$_{114}Fl$$
$$\left[ {Rn} \right]5{f^{14}}6{d^{10}}7{s^2}7{p^2}$$
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(a)
554. Why do boron and aluminium halides behave as Lewis acids?
A
Both halides $$\left( {M{X_3}} \right)$$ can accept electrons from a donor to complete their octet.
B
Both halides $$\left( {M{X_3}} \right)$$ can donate a pair of electrons.
C
Both halides $$\left( {M{X_3}} \right)$$ are covalent polymeric structures.
D
Both halides $$\left( {M{X_3}} \right)$$ react with water to give hydroxides and $$HCl.$$
Answer :
Both halides $$\left( {M{X_3}} \right)$$ can accept electrons from a donor to complete their octet.
555. Which of the following is coloured
A
$$NO$$
B
$${N_2}O$$
C
$$S{O_3}$$
D
$${\text{None }}$$
Answer :
$${\text{None }}$$
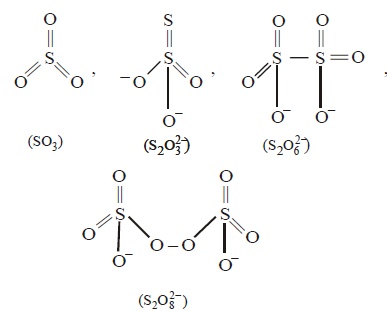
556. The number of $$S - S$$ bonds in $$S{O_3},{S_2}O_3^{2 - },{S_2}O_6^{2 - }$$ and $${S_2}O_8^{2 - }$$ respectively are
A
1, 0, 0, 1
B
1, 0, 1, 0
C
0, 1, 1, 0
D
0, 1, 0, 1
Answer :
0, 1, 1, 0
557. What happens when silicon is heated with methyl chloride in presence of copper as a catalyst at $$573\,K?$$
A
Methyl substituted chlorosilanes are formed.
B
Only $$M{e_4}Si$$ is formed.
C
Polymerised chains of $${\left( {C{H_3}} \right)_3}SiCl$$ are formed.
D
Silicones are formed.
Answer :
Methyl substituted chlorosilanes are formed.
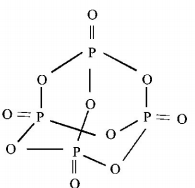
558. Number of sigma bonds in $${P_4}{O_{10}}$$ is
A
$$6$$
B
$$7$$
C
$$17$$
D
$$16$$
Answer :
$$16$$
559.
Match the column I with column II and mark the appropriate choice.
Column I
Column II
a.
$${H_2}S{O_3}$$
1.
+6, dibasic
b.
$${H_2}S{O_5}$$
2.
+5, dibasic
c.
$${H_2}{S_2}{O_6}$$
3.
+6, monobasic
d.
$${H_2}S{O_4}$$
4.
+4, dibasic
A
a - 1, b - 2, c - 3, d - 4
B
a - 2, b - 3, c - 1, d - 4
C
a - 3, b - 4, c - 2, d - 1
D
a - 4, b - 3, c - 2, d - 1
Answer :
a - 4, b - 3, c - 2, d - 1
560. Which of the following fluorides does not exist?
A
$$N{F_5}$$
B
$$P{F_5}$$
C
$$As{F_5}$$
D
$$Sb{F_5}$$
Answer :
$$N{F_5}$$