541. Elements of which of the following groups will form anions most readily?
A
Oxygen family
B
Nitrogen family
C
Halogens
D
Alkali metals
Answer :
Halogens
542. Which one of the following anions is present in the chain structure silicates?
A
$$S{i_2}O_7^{6 - }$$
B
$${\left( {S{i_2}O_5^{2 - }} \right)_n}$$
C
$${\left( {SiO_3^{2 - }} \right)_n}$$
D
$$SiO_4^{4 - }$$
Answer :
$${\left( {SiO_3^{2 - }} \right)_n}$$
543. In nitrogen family, the $$H - M - H$$ bond angle in the hydrides gradually becomes closer to $${90^ \circ }$$ on going from $$N$$ to $$Sb.$$ This shows that gradually
A
the basic strength of the hydrides increases.
B
almost pure $$p$$ - orbitals are used for $$M-H$$ bonding.
C
the bond energies of $$M-H$$ bonds increases.
D
the bond pairs of electrons become nearer to the central atom.
Answer :
almost pure $$p$$ - orbitals are used for $$M-H$$ bonding.
544. Which of the following compounds is not matched correctly with its structure?
A
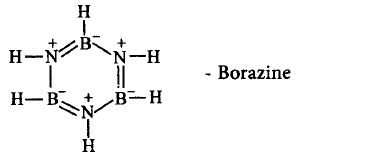

B
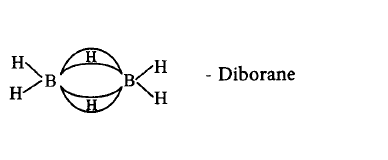

C
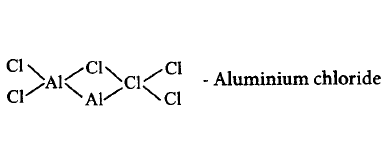

D


Answer :


545. Phosphine is not obtained by which of the following reaction ?
A
White $$P$$ is heated with $$NaOH.$$
B
Red $$P$$ is heated with $$NaOH.$$
C
$$C{a_3}{P_2}$$ reacts with water.
D
Phosphorus trioxide is boiled with water.
Answer :
Red $$P$$ is heated with $$NaOH.$$
546. For making good quality mirrors, plates of float glass are used. These are obtained by floating molten glass over a liquid metal which does not solidify before glass. The metal used can be
A
tin
B
sodium
C
magnesium
D
mercury
Answer :
mercury
547. The percentage of $$\pi$$-character in the orbitals forming $$P - P$$ bonds in $${P_4}$$ is
A
25
B
33
C
50
D
75
Answer :
75
548. Fluorine is the best oxidising agent because it has
A
highest electron affinity
B
highest reduction potential
C
highest oxidation potential
D
lowest electron affinity
Answer :
highest reduction potential
549. State the hybridisation of $$C$$ atom in graphite.
A
$$s{p^3}$$
B
$$sp$$
C
$$s{p^2}$$
D
$${\text{None of these}}$$
Answer :
$$s{p^2}$$
550. An aqueous solution of boric acid is found to be weakly acidic in nature because
A
it is a protic acid which donates protons in aqueous solution.
B
it is a Lewis acid which abstracts $$O{H^ - }$$ from water and leaves $${H^ + }$$ to make the solution acidic.
C
it gives metaboric acid when dissolved in water.
D
it is prepared by reaction of borax with sulphuric acid hence it behaves as an acid.
Answer :
it is a Lewis acid which abstracts $$O{H^ - }$$ from water and leaves $${H^ + }$$ to make the solution acidic.