501. Which of the following compounds are formed when $$BC{l_3}$$ is treated with water?
A
$${H_3}B{O_3}$$
B
$${B_2}{H_6}$$
C
$${B_2}{O_3}$$
D
$$HB{O_2}$$
Answer :
$${H_3}B{O_3}$$
502. Aluminium oxide is not reduced by chemical reactions due to
A
its highly stable nature
B
its highly unstable nature
C
its amphoteric nature
D
its highly explosive nature
Answer :
its highly stable nature
503.
Match the column I with column II and mark the appropriate choice.
Column I
Column II
a.
Borax-bead
1.
Alum
b.
Inorganic benzene
2.
Diborane
c.
Antiseptic
3.
Metaborate
d.
Bridged hydrogens
4.
Borazine
A
a - 1, b - 3, c - 2, d - 4
B
a - 3, b - 4, c - 1, d - 2
C
a - 4, b - 3, c - 1, d - 2
D
a - 2, b - 3, c - 4, d - 1
Answer :
a - 3, b - 4, c - 1, d - 2
504. In which of the following pairs, both the species are not isostructural?
A
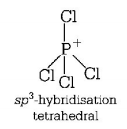
$$SiC{l_4},PCl_4^ + $$
B
$${\text{Diamond, carbide}}$$
C
$$N{H_3},P{H_3}$$
D
$$Xe{F_4},Xe{O_4}$$
Answer :
$$Xe{F_4},Xe{O_4}$$
505. Quartz is extensively used as a piezoelectric material, it contains __________.
A
$$Pb$$
B
$$Si$$
C
$$Ti$$
D
$$Sn$$
Answer :
$$Si$$
506. Incorrect statement about $$P{H_3}$$ is :
A
It is produced by hydrolysis of $$C{a_3}{P_2}.$$
B
It gives black $$ppt.\left( {C{u_3}{P_2}} \right)$$ with $$CuS{O_4}$$ solution.
C
Spontaneously burns in presence of $${P_2}{H_4}.$$
D
It does not react with $${B_2}{H_6}.$$
Answer :
It does not react with $${B_2}{H_6}.$$
507. Group 13 elements show
A
only + 1 oxidation state
B
only + 3 oxidation state
C
+ 1 and + 3 oxidation states
D
+ 1, + 2 and + 3 oxidation states
Answer :
+ 1 and + 3 oxidation states
508. Which one of the following oxides of chlorine is obtained by passing dry chlorine over silver chlorate at $${90^ \circ }C?$$
A
$$C{l_2}O$$
B
$$Cl{O_3}$$
C
$$Cl{O_2}$$
D
$$Cl{O_4}$$
Answer :
$$Cl{O_2}$$
509. Which of the following statements is not correct for $$S{O_2}$$ gas?
A
It acts as bleaching agent in moist conditions.
B
Its dilute solution is used as disinfectant.
C
Its molecules have linear geometry.
D
Acidified $$KMn{O_4}$$ is decolourised when $$S{O_2}$$ is passed through it.
Answer :
Its molecules have linear geometry.
510. In nitroprusside ion, the iron and $$NO$$ exist as $$F{e^{2 + }}$$ and $$N{O^ + }$$ rather than $$F{e^{3 + }}$$ and $$NO.$$ These forms can be differentiated by
A
estimating the concentration of iron.
B
measuring the concentration of $$C{N^ - }.$$
C
measuring the solid state magnetic moment.
D
thermally decomposing the compound.
Answer :
measuring the solid state magnetic moment.
.PNG)
.PNG)
.PNG)
.PNG)
.PNG)