21. In the preparation of compounds of $$Xe,$$ Bartlett had taken $$O_2^ + \,PtF_6^ - $$ as a base compound. This is because
A
both $${O_2}$$ and $$Xe$$ have same size
B
both $${O_2}$$ and $$Xe$$ have same electron gain enthalpy
C
both $${O_2}$$ and $$Xe$$ have almost same ionisation enthalpy
D
both $$Xe$$ and $${O_2}$$ are gasess
Answer :
both $${O_2}$$ and $$Xe$$ have almost same ionisation enthalpy
22. The compound which gives off oxygen on moderate heating is :
A
cupric oxide
B
mercuric oxide
C
zinc oxide
D
aluminium oxide
Answer :
mercuric oxide
23. Iron sulphide is heated in air to form $$A,$$ an oxide of sulphur. $$A$$ is dissolved in water to give an acid. The basicity of this acid is
A
2
B
3
C
1
D
Zero
Answer :
2
24. Interhalogen compounds are more reactive than the individual halogens because
A
they are prepared by direct combination of halogens
B
$$X - X'$$ bond is weaker than $$X - X$$ or $$X' - X'$$ bonds
C
they are thermally more stable than halogens
D
there is a large difference in their electronegativity
Answer :
$$X - X'$$ bond is weaker than $$X - X$$ or $$X' - X'$$ bonds
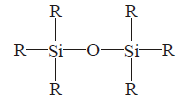
25. Identify the incorrect statement :
A
In $${\left( {S{i_3}{O_9}} \right)^{6 - }},$$ tetrahedral $$Si{O_4}$$ units share two oxygen atoms.
B
Trialkylchlorosilane on hydrolysis gives $${R_3}SiOH.$$
C
$$SiC{l_4}$$ undergoes hydrolysis to give $${H_4}Si{O_4}.$$
D
$${\left( {S{i_3}{O_9}} \right)^{6 - }}$$ has cyclic structure.
Answer :
Trialkylchlorosilane on hydrolysis gives $${R_3}SiOH.$$
26. Boron cannot form which one of the following anions?
A
$$BF_6^{3 - }$$
B
$$BH_4^ - $$
C
$$B\left( {OH} \right)_4^ - $$
D
$$BO_2^ - $$
Answer :
$$BF_6^{3 - }$$
27. The gases respectively absorbed by alkaline pyrogallol and oil of cinnamon are
A
$${O_3},C{H_4}$$
B
$${O_2},{O_3}$$
C
$$S{O_2},C{H_4}$$
D
$${N_2}O,{O_3}$$
Answer :
$${O_2},{O_3}$$
28. Polyanion formation is maximum in
A
nitrogen
B
oxygen
C
sulphur
D
boron
Answer :
sulphur
29. $$Al$$ is more reactive than $$Fe$$ but $$Al$$ is less easily corroded than $$Fe$$ because
A
it is a noble metal
B
oxygen forms a protective oxide layer
C
iron undergoes reaction easily with water
D
$$Fe$$ form mono and divalent ions.
Answer :
oxygen forms a protective oxide layer
30. Which amongst the following is the strongest acid?
A
$$CHB{r_3}$$
B
$$CH{l_3}$$
C
$$CH{\left( {CN} \right)_3}$$
D
$$CHC{l_3}$$
Answer :
$$CH{\left( {CN} \right)_3}$$