21. The reason for the stability of $$G{d^{3 + }}\,ion$$ is
A
$$4f$$ subshell - half filled.
B
$$4f$$ subshell - completely filled.
C
Possesses the general electronic configuration of noble gases.
D
$$4f$$ subshell empty.
Answer :
$$4f$$ subshell - half filled.
22. $$Zn$$ gives $${H_2}$$ gas with $${H_2}S{O_4}$$ and $$HCl$$ but not with $$HN{O_3}$$ because
A
$$Zn$$ acts as an oxidising agent when react with $$HN{O_3}.$$
B
$$HN{O_3}$$ is weaker acid than $${H_2}S{O_4}$$ and $$HCl.$$
C
In electrochemical series $$Zn$$ is above hydrogen.
D
$$NO_3^ - \,ion$$ is reduced in preference to hydroniumion.
Answer :
$$NO_3^ - \,ion$$ is reduced in preference to hydroniumion.
23. The electronic configuration of gadolinium ( $$At.$$ No. 64 ) is
A
$$\left[ {Xe} \right]4{f^8}5{d^1}6{s^2}$$
B
$$\left[ {Xe} \right]4{f^7}5{d^1}6{s^2}$$
C
$$\left[ {Xe} \right]4{f^3}5{d^5}6{s^2}$$
D
$$\left[ {Xe} \right]4{f^6}5{d^2}6{s^2}$$
Answer :
$$\left[ {Xe} \right]4{f^7}5{d^1}6{s^2}$$
24.
Which of the following lanthanoid ions is diamagnetic?
$$\left( {{\text{At}}{\text{.}}\,{\text{no}}{\text{.}}\,Ce = 58,Sm = 62,} \right.$$ $$\left. {Eu = 63,Yb = 70} \right)$$
A
$$C{e^{2 + }}$$
B
$$S{m^{2 + }}$$
C
$$E{u^{2 + }}$$
D
$$Y{b^{2 + }}$$
Answer :
$$Y{b^{2 + }}$$
25. The number of unpaired electrons in gaseous species of $$M{n^{3 + }},C{r^{3 + }}$$ and $${V^{3 + }}$$ respectively are ______ and the most stable species is ______ .
A
4, 3 and 2 ; $${V^{3 + }}$$
B
3, 3 and 2 ; $$C{r^{3 + }}$$
C
4, 3 and 2 ; $$C{r^{3 + }}$$
D
3, 3 and 3 ; $$M{n^{3 + }}$$
Answer :
4, 3 and 2 ; $$C{r^{3 + }}$$
26. The correct electronic configuration of $$f$$ - block elements is
A
$$\left( {n - 2} \right){f^{1 - 14}}\left( {n - 1} \right){d^{0 - 1}}\,n{s^2}$$
B
$$\left( {n - 1} \right){f^{1 - 14}}\left( {n - 1} \right){d^{0 - 1}}\,n{s^2}$$
C
$$\left( {n - 3} \right){f^{1 - 14}}\left( {n - 2} \right){d^{0 - 1}}\left( {n - 1} \right){s^2}$$
D
$$\left( {n - 2} \right){f^{0 - 1}}\left( {n - 1} \right){d^{0 - 1}}\,n{s^1}$$
Answer :
$$\left( {n - 2} \right){f^{1 - 14}}\left( {n - 1} \right){d^{0 - 1}}\,n{s^2}$$
27. The salts of $$Cu$$ in +1 oxidation state are unstable because
A
$$C{u^ + }$$ has $$3{d^{10}}$$ configuration
B
$$C{u^ + }$$ disproportionates easily to $$Cu\left( 0 \right)$$ and $$C{u^{2 + }}$$
C
$$C{u^ + }$$ disproportionates easily to $$C{u^{2 + }}$$ and $$C{u^{3 + }}$$
D
$$C{u^ + }$$ is easily reduced to $$C{u^{2 + }}$$
Answer :
$$C{u^ + }$$ disproportionates easily to $$Cu\left( 0 \right)$$ and $$C{u^{2 + }}$$
28. The blue complex formed on addition of $$conc.\,N{H_4}OH$$ solution to a $$C{u^{2 + }}$$ salt solution has the structure ?
A
$${\left[ {Cu{{\left( {N{H_4}} \right)}_4}} \right]^{2 + }}$$
B
$${\left[ {Cu{{\left( {N{H_3}} \right)}_2}} \right]^{2 + }}$$
C
$${\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}$$
D
$${\left[ {Cu{{\left( {N{H_4}} \right)}_2}} \right]^{2 + }}$$
Answer :
$${\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}$$
29.
The $$d$$-electron configurations of $$C{r^{2 + }},M{n^{2 + }}\,F{e^{2 + }}$$ and $$C{o^{2 + }}$$ are $${d^4},{d^5},{d^6}$$ and $${d^7}$$ respectively. Which one of the following will exhibit minimum paramagnetic behaviour?
$$\left( {{\text{At}}{\text{.}}\,{\text{no}}{\text{.}}\,Cr = 24,Mn = 25,} \right.$$ $$\left. {Fe = 26,Co = 27} \right)$$
A
$${\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
B
$${\left[ {Co{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
C
$${\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
D
$${\left[ {Mn{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
Answer :
$${\left[ {Co{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
30. Which compound is formed when excess of $$KCN$$ is added to aqueous solution of copper sulphate?
A
$$Cu{\left( {CN} \right)_2}$$
B
$${K_2}\left[ {Cu{{\left( {CN} \right)}_4}} \right]$$
C
$$K\left[ {Cu{{\left( {CN} \right)}_2}} \right]$$
D
$${K_3}\left[ {Cu{{\left( {CN} \right)}_4}} \right]$$
Answer :
$${K_3}\left[ {Cu{{\left( {CN} \right)}_4}} \right]$$
 ($$4$$ unpaired electrons)
($$4$$ unpaired electrons)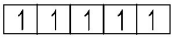 ($$5$$ unpaired electrons)
($$5$$ unpaired electrons) ($$4$$ unpaired electrons)
($$4$$ unpaired electrons) ($$3$$ unpaired electrons)
($$3$$ unpaired electrons)