Question
Arrange the following in decreasing order of solubility in water
Arrange the following in decreasing order of solubility in water
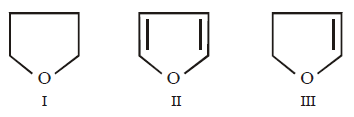
A.
I > III > II
B.
III > II > I
C.
II > III > I
D.
All are equally soluble
Answer :
I > III > II
Solution :
Higher the electron density on $$O,$$ stronger is the $$H$$ - bond with water and thus more is the solubility. Thus solubility of the three ethers follow the order
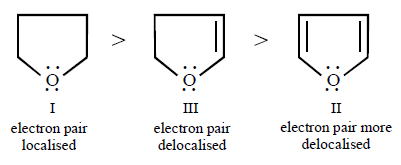
Higher the electron density on $$O,$$ stronger is the $$H$$ - bond with water and thus more is the solubility. Thus solubility of the three ethers follow the order
