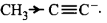Question
Arrange the following carbanions in order of their decreasing stability.
Arrange the following carbanions in order of their decreasing stability.
$$\eqalign{
& \left( {\text{i}} \right){H_3}C - C \equiv {C^ - } \cr
& \left( {{\text{ii}}} \right)H - C \equiv {C^ - } \cr
& \left( {{\text{iii}}} \right){H_3}C - CH_2^ - \cr} $$
A.
(i) > (ii) > (iii)
B.
(ii) > (i) > (iii)
C.
(iii) > (ii) > (i)
D.
(iii) > (i) > (ii)
Answer :
(ii) > (i) > (iii)
Solution :
The order of decreasing stability of carbanions is :
$$\mathop {H - C \equiv {C^ - }}\limits_{\left( {{\text{ii}}} \right)} > \mathop {C{H_3} - C \equiv {C^ - }}\limits_{\left( {\text{i}} \right)} $$ $$ > \mathop {C{H_3} - CH_2^ - }\limits_{\left( {{\text{iii}}} \right)} $$
$$sp$$ - hybridised carbon atom is more electronegative than $$s{p^3}$$ - hybridised carbon atom and hence, can accommodate the negative charge more effectively. $$ - C{H_3}$$ group has $$+I$$ effect, therefore, it intensifies the negative charge and hence, destabilises the carbanion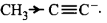
The order of decreasing stability of carbanions is :
$$\mathop {H - C \equiv {C^ - }}\limits_{\left( {{\text{ii}}} \right)} > \mathop {C{H_3} - C \equiv {C^ - }}\limits_{\left( {\text{i}} \right)} $$ $$ > \mathop {C{H_3} - CH_2^ - }\limits_{\left( {{\text{iii}}} \right)} $$
$$sp$$ - hybridised carbon atom is more electronegative than $$s{p^3}$$ - hybridised carbon atom and hence, can accommodate the negative charge more effectively. $$ - C{H_3}$$ group has $$+I$$ effect, therefore, it intensifies the negative charge and hence, destabilises the carbanion